Objective: To evaluate if the hemostatic high-dose aprotinin seems to reduce the inflammatory process after extracorporeal circulation (ECC) in children.
Methods: A prospective randomized study was conducted on children aged 30 days to 4 years submitted to correction of acyanogenic congenital heart disease with ECC and divided into two groups: Control (n=9) and Aprotinin (n=10). In the Aprotinin Group the drug was administered before and during ECC and the systemic inflammatory response and hemostatic and multiorgan dysfunctions were analyzed on the basis of clinical and biochemical markers. Differences were considered to be significant when P<0.05. Results: The groups were similar regarding demographic and intraoperative variables, except for a greater hemodilution in the Aprotinin Group. The drug had no benefit regarding time of mechanical pulmonary ventilation, permanence in the postoperative ICU and length of hospitalization, or regarding the use of inotropic drugs and renal function. The partial arterial oxygen pressure/inspired oxygen fraction ratio (PaO2/FiO2) was significantly reduced 24 h after surgery in the Control Group. Blood loss was similar for both groups. Significant leukopenia was observed in the Aprotinin Group during ECC, followed by leukocytosis. Tumor necrosis factor alpha (TNF- á), interleukins (IL)-6, IL-8, IL-10, IL-6/IL-10 ratio did not differ significantly between groups. The postoperative IL-6/IL-10 fraction increased significantly in the Control Group. There were no complications with the use of aprotinin. Conclusion: In this series, hemostatic high-dose aprotinin did not minimize the clinical manifestations or serum markers of the inflammatory systemic response.
Objetivo: Avaliar se a aprotinina em altas doses hemostáticas pode reduzir o processo inflamatório após circulação extracorpórea (CEC) em crianças. Métodos: Estudo prospectivo randomizado em crianças de 30 dias a 4 anos de idade, submetidas à correção de cardiopatia congênita acianogênica, com CEC e divididas em dois grupos, um denominado Controle (n=9) e o outro, Aprotinina (n=10). Neste, o fármaco foi administrado antes e durante a CEC. A resposta inflamatória sistêmica e disfunções hemostática e multiorgânicas foram analisadas por marcadores clínicos e bioquímicos. Foram consideradas significantes as diferenças com P<0,05. Resultados: Os grupos foram semelhantes quanto às variáveis demográficas e intra-operatórias, exceto por maior hemodiluição no Grupo Aprotinina. Não houve benefício quanto aos tempos de ventilação pulmonar mecânica, permanência no CTIP e hospitalar, nem quanto ao uso de inotrópicos e função renal. A relação PaO2/FiO2 (pressão parcial de oxigênio arterial/fração inspirada de oxigênio) apresentou queda significativa com 24 h pós-operatório, no Grupo Controle. As perdas sanguíneas foram semelhantes nos dois grupos. No grupo Aprotinina surgiu leucopenia significativa, em CEC, seguida de leucocitose. Fator de necrose tumoral alfa (TNF -a), Interleucinas (IL)-6, IL-8, IL-10, proporção IL-6/IL-10 não apresentaram diferenças marcantes intergrupos. A proporção IL-6/IL-10 PO aumentou no grupo Controle. Não houve complicações com o uso da aprotinina. Conclusão: Nesta casuística, a Aprotinina em altas doses hemostáticas não minimizou as manifestações clínicas e os marcadores séricos de resposta inflamatória sistêmica.
INTRODUCTION
The morbidity and mortality of cardiopulmonary bypass (CPB) occurs, in large part to the limited biocompatibility of materials, triggering a systemic multiorgan post-infusion dysfunction, expressed by myocardial depression, vasomotor dysfunction, respiratory, renal and hepatic failure, cognitive and thermal regulation disorders, and bleeding due to coagulopathy, characterizing the systemic inflammatory response syndrome (SIRS)[1]. Newborns and infants are susceptible to systemic capillary leakage syndrome (SCLS), characterized by severe tissue edema, pericardial or pleural effusion and/or ascites. These syndromes result from the release of cytokines, histamine, bradykinin and other vasoactive agents, and leukocyte activation, endothelial, platelet, parenchymal cells, systems contact (kallikrein), complement and coagulation/fibrinolysis [2].
Although some patients, mostly adults recover well, children of low body weight, particularly newborns and infants often present complications due to systemic inflammatory reaction. These may determine exposure to a larger number of donor of blood products, prolonged hemostasis in the operating room, need to late sternal closure, with consequent prolongation of mechanical ventilation and stay in the Pediatric Intensive Care Unit (PICU), and worrying morbidity and mortality associated with high hospital costs. Strategies to anticipate them, avoid them or fight them are of great interest, especially the preoperative scores of risk stratification, the anesthetic and surgical refinement and optimization of CPB circuits [3].
The use of aprotinin, a nonspecific inhibitor of serine proteases consisting of the polypeptide chain of 6512 Daltons, hydrophilic and basic, was one of the pharmacological strategies for reducing the inflammatory response, which property reduces hemostatic blood loss after CPB. The activation of coagulation, fibrinolytic and inflammatory networks is mediated by serine proteases [4]. At the hemostatic doses, inflammatory effect of aprotinin includes the maintenance of platelet function, lower release of elastase and complement activation, inhibition of up-regulation of adhesion molecules of monocytes and granulocytes and decreased endothelial transmigration of neutrophils. It also decreases the plasma concentration of IL-1β, IL-6, IL-8, and TNF-α and IL-8 in the airways, increasing the serum level of IL-10 [3,4].
Pediatric studies with aprotinin fail in the difficulty to interpret them [5] due to the wide variation in dosage, the great variability of metabolism and antifibrinolytic action of aprotinin in neonates, hemodilution, the dispersion in age and types of heart disease in the cohort studied and the different surgical procedures performed, with consequent variability in CPB and surgery [5,6], facts inherent in the population undergoing cardiac surgery.
The benefits related to the antiinflammatory effects of aprotinin in children are inaccurate and scarce, reporting a reduction of transpulmonary pressure gradients after cavo-pulmonary anastomosis [7] and reduction of postoperative inotropic needs [8]. Mössinger et al. [6] in 2003 demonstrated that this drug reduces rates of lung and hemostatic dysfunctions, but does not alter serum inflammatory markers.
It was not demonstrated a higher incidence of myocardial infarction (MI), acute renal failure (ARF) and cerebrovascular accident (CVA) with aprotinin in adults until a non-randomized observational study that reported the opposite, also establishing a higher mortality at 5 years after surgery. The manufacture of aprotinin was suspended in November 2007. Results from the Canadian BART study (
Blood conservation using Anti-fibrinolytics: a Randomized Trial in a cardiac surgery population study) suggest increased risk of mortality [9,10].
However, in recent retrospective cohort studies of children who had undergone surgery with CPB, there was no association between use of aprotinin and renal failure, dialysis, neurologic complications and mortality [11], and reported that the CPB time over 100 minutes was the main marker of postoperative renal dysfunction in neonates but not the use of the drug, which was not associated with this risk in the immediate postoperative period [12,13]. The use of safety data in adults for pediatric practice requires caution and is of questionable validity, since they are two distinct populations with distinct risk factors, and drugs that have the greatest risk of complications in adults can be safe in children, and vice versa. The outlook for future randomized controlled pediatric studies with aprotinin proved to be limited up tp now [10].
On this basis, since there is a temporary suspension of production of aprotinin associated with the fact of not reporting significant adverse effects in children, especially renal failure, and the fact that there is little information available about its influence on the systemic inflammatory reaction in children when used with haemostatic purpose, which still arouses intense current scientific interest, this study was performed in an attempt to demonstrate the anti-inflammatory action of aprotinin in high doses in a subgroup of acyanotic children undergoing surgery in our institution.
The aim of this study was to investigate the antiinflammatory effects of intraoperative administration of aprotinin in high doses in children undergoing correction of acyanogenic congenital heart disease with CPB.
METHODS
Patients
We studied 19 children of both genders, undergoing correction of acyanogenic congenital heart disease with CPB from January to December 2004. The patients were divided randomly into two groups: aprotinin (n=10) and control group (n=9). The study was prospective, randomized and not blinded. The inclusion criteria were non-emergency surgeries and aged between one month and four years. The exclusion criteria were: previous cardiovascular surgery, exposure to aprotinin within six months prior to surgery, use of salicylates until 7 days before surgery, allergy and immunological disorders, hepatic, renal or coagulation disorders and episodes of cardiac arrest, sepsis and vasculitis, less than two months before surgery. Parents or legal guardians signed written informed consent. The study was approved by the Research Ethics Committee of the Clinics Hospital, Faculty of Medicine of Ribeirão Preto, University of São Paulo (HC FMRP-USP), under No. 6665/2004.
Methodology
The distribution of patients in both groups was performed by drawing lots, after induction of anesthesia.
Anesthetic and surgical techniques
In the operating room, the children were placed in the supine position on thermal mattress and under a flow of hot air. They were monitored with electrocardiogram (ECG) and pulse oximetry. The induction and anesthetic plan consisted of midazolam, fentanyl or sufentanil associated with inhalation of isoflurane and muscle relaxation with vecuronium or pancuronium. After tracheal intubation, mechanical ventilation was started. Invasive monitoring of arterial pressure (AP), central venous pressure (CVP), temperatures (oropharyngeal and peripheral) and urine output was similar among children. Corticosteroids were not used. After induction, we administered amikacin (7.5mg/kg intravenously) associated with cefazolin (40mg/kg intravenously) in children admitted in less than 48 hours. In the other, the association was performed with vancomycin (10mg/kg intravenously). Additional dose of vancomycin (5mg/kg) was added to the reservoir of the oxygenator.
Aprotinin (Trasylol®, Bayer, Leverkusen, Germany) at a dose of 240 mg/m
2 was infused endovenously for 20-30 min, from the beginning of the surgical incision, followed by continuous infusion of aprotinin, 56mg/m
2/h up to complete the dressing. The drug (240 mg/m
2) was also added to the perfusate oxygenator. Ten minutes before the infusion of aprotinin a sensitivity test is performed with 10,000 minidose UIC (kallikrein inhibitor units) endovenously. Under topical antisepsis with alcohol povidone solution, median sternotomy and total thymectomy were performed. Children were heparinized (3mg/kg, intravenously)(Heparin sodium, Roche, Basle, Switzerland), under control of the Activated Clotting Time (ACT) (Hemotec ACT II ®, Medtronic, Englewood, CO, USA), so that to maintain ACT above 480 seconds in both groups (with additional doses of heparin, 0.5mg/kg intravenously).
The ascending aorta and vena cava were cannulated. In CEC, performed with passive venous drainage, we used a hollow fiber capillary membrane oxygenator - mainly Polystan Safe Mini or Micro (Maquet Gmbh & Co, KG, Medical Systems Com. Ind. Medica Ltda., SP, Brazil) or D901 Lilliput 1 DIDECO (Cobe CV, Sorin Group Company, Mirandola, Italy), with their respective arterial filters. Roller pumps were used with blood flow in normothermia, 2.5 l/m
2/min. The oropharyngeal temperature in CPB was reduced to 28ºC through thermal change in the oxygenator. The perfusate was calculated to yield a hematocrit of 30% and consisted of red blood cells (RBC), Ringer's solution, fresh frozen plasma, 20% Mannitol (4-5ml/kg), sodium heparin 1 mg/10 ml of blood derivatives and bicarbonate 8.4% sodium 1mEq/kg. In the aprotinin group the volume of drugs added to the perfusate was included in the calculation. Hemoconcentrators with polyarylethersulphone membrane were used for ultrafiltration, which was started in the reheating.
The hematocrit during CPB was maintained by addition of RBC. After aortic clamping, anterograde hyperkalemic cardioplegia (10ml/kg) at 4ºC, suspended at one meter height in relation to the operating table, was passively infused in the aortic root and repeated every 30 min. The first dose was crystalloid followed by bood doses. The systemic rewarming, up to 37ºC, was started simultaneously with the infusion (0.5 to 1.5mg/kg/min) of sodium nitroprusside. After completion of CPB, the aortic and superior vena cava cannulas were removed, and the arterial line was connected to the inferior vena cava cannula. The volume in the oxygenator was ultrafiltrated in the intervals between replacements of volume infused by cannula of inferior vena cava. It was administered protamine hydrochloride (ICN Farmacêutica Ltda., Valeant Pharmaceuticals International, USA), 1:1 ratio in relation to the total dose of heparin used, and it was confirmed the return of ACT to baseline levels. The remaining blood in the CPB circuit was recovered in bags for transfer without anticoagulant for subsequent decannulation, endovenous infusion by intravenous catheter. The pericardium was closed, if not generating hemodynamic instability. Mediastinal drain. Closure in layers.
Preoperative clinical characteristics
We analyzed demographic variables (age, gender, weight, height and body surface) and calculated the risk category according to RACSH-1 and Aristotle Basic scores, as well as the modified Ross and Reithmann scores, for congestive heart failure (CHF), since they may influence the inflammatory response. The medications in use, presence of arrhythmias, electrocardiography, chest radiography, echocardiography and cardiac catheterization and surgical diagnosis were recorded. Hemodynamic parameters were also recorded (pulmonary arterial and systemic pressures), and obtained complete blood count, coagulation and renal function tests (urea and serum creatinine) and hepatic tests (glutamic-oxalacetic transaminase - SGOT and bilirubin), as markers of organ dysfunction resulting from SIRS and/or SCLS.
Surgical data
Surgeries performed were recorded, intracardiac access and variable of duration of surgery, anesthesia and CPB, aortic clamping time, minimum temperature in the oropharynx, fluid balance, urine output, volume of packed red cells and fresh frozen plasma and platelet concentrate , ACT before, during and after CPB and complications.
Postoperative clinical status
The postoperative management followed a preset protocol for the team. On arrival at the PICU, we calculated the PRISM score (Pediatric Risk Index Score for Mortality). The criteria for assessing systemic inflammatory response (SIRS and SCLS)[1,2,14] have been checked within 48 hours. Criteria for assessing the hemodynamic, respiratory and metabolic presentations, Glasgow coma scale and liver dysfunction were computed [14], as well as postoperative complications.
Retrospectively, we calculated the index recently proposed by Mattos et al. [15] in 2006. The times of use of inotropic and EV vasoactive drugs, time of use of nitric oxide, and duration of stay in PICU in adittion to the elapsed until discharge or death were measured, as well as the duration of mechanical ventilation (MV). The cumulative postoperative bleeding in 4, 12, 24 and 48 h, and the use of blood products, at 6 and 24 h postoperatively, were expressed in ml/kg, and the number of donors that patients were exposed to was recorded. Renal failure was assessed by the volume of urine output, dosage of urea and blood creatinine and estimated creatinine clearance (Schwartz formula).
Biochemical and hematological assessment
Arterial blood samples (3 ml) were collected at the following times: T1: After induction of anesthesia, before the administration of aprotinin T2 - 15 minutes after initiation of CPB, T3 - Immediately before the end of CPB, T4 - Five minutes after protamine administration; T5 - 48 hours after T4, T6 - 12 hours after T4, T7 - 24 hours after T4, T8 - 48 hours after T4.
The counts of leukocytes and platelets were observed in T1 to T8. Gasometry and lactate levels were determined in arterial blood (860 Rapilab analyzer, Bayer AG, Leverkusen, Germany), in T1 to T8.
As for the dosage of inflammatory markers, 3ml of blood from each sample were preserved in tubes without anticoagulant, with separator gel (BD Vacutainer Systems®, Belliver Industrial State, Plymouth, UK) at 4ºC, and then centrifuged at the same temperature (10 min, 3000 rpm). Serum was pipetted and stored at -70ºC in Eppendorf tubes for further processing by enzyme immunoassay (ELISA), for measurement of TNF-α (tumor necrosis factor alpha), IL (interleukin)-6 , IL-8 and IL-10 (BD OptEIA set and kit II, BD Biosciences Pharmingen, San Diego, CA, USA) at 150 ìl aliquots of plasma or more. The analysis was performed at the Laboratory of Endocrinology, HC-FMRP-USP. The kinetic profile of serum levels of cytokines TNF-α, IL-6, IL-8 and IL-10 were quantified and corrected for hemodilution by serum albumin. Albumin was measured in plasma by colorimetric method, in Bausch & Lomb spectrophotometer (Model Spectronic 70.1, USA) in T1 to Tn (T2 to T4) (normal value 3.5 to 5.5 g/dl). The correction was performed using the following formula:
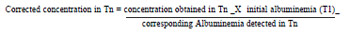
Qualitative variables were expressed in absolute and relative frequencies. Quantitative variables were expressed by the minimum and maximum values, mean, median, standard deviation and quartiles. The study presents measurable continuous variables placed on a scale and categorized into two groups and eight times. As independent variable, we have the treatment with aprotinin and the times in which measures of the dependent variables were taken (T1 to T8). For analysis, intergroup comparison of variables for analysis, particularly cytokines, leukocyte count, and hemoglobin and hematocrit, was performed using mixed-effects model for each dependent variable (fixed and random effects), where the responses of the same individual are grouped (PROC MIXED ® pack, SAS/STAT®, version 9, SAS Institute Inc., Cary, NC, USA), since it was considered a normal distribution. In order to allow that the assumptions associated with the proposed model were fully met, some logarithmic or Box-Cox transformations were used and are indicated in the results when they are presented. The model is similar to ANOVA, but as it works with people, we used the random effect (assuming that the sample is of the population, in the case of this study, children who underwent surgery in our hospital) and the response of each individual (which is particular). The model worked simultaneously on each response each time. As the assumption zero waste, and constant variance were tested and satisfied, the data could be described by the normal distribution, considering valid the parametric method. It was also used the Pearson correlation coefficient (PROC CORR®, SAS/STAT® software, version 9, SAS Institute Inc., Cary, NC, USA) in order to examine correlations between cytokines variables.
In comparisons of subgroups, as well as in intra- and intergroups in the other variables, we used the nonparametric exact test of Wilcoxon (Software R Development Core Team (2005). R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051 -07-0, URL http://www.R-project.org) for two independent samples, which are indicated in the results.
In the figures depicting boxes, the horizontal limits correspond to 25th and the 75th percentiles, the line inside the box represents the median and the limits and lines outside the boxes reflect the variance of the variable. The full point expresses the mean, and empty points the outliers.
P values < 0.05 were considered statistically significant.
RESULTS
In comparison between means and standard deviations of the groups, those from the aprotinin group will precede. In figures, the significant differences intragroups, in relation to T1, are indicated by *, and intergroup by **.
Preoperative
As our service is in general tertiary hospital, most children, even electively, remained variable time of hospital stay before surgery. The groups which were possible to obtain during the period were similar in age and anthropometric variables (Table 1). Except for modified Ross score, slightly more pronounced in the aprotinin group (median 5.5 vs. 3) the RACHS-1 score (median 2) and Aristotle Basic (median 6) were similar in both groups (Table 2). Cardiac malformations were similar in both groups (Table 3) as well as surgeries performed. The groups were considered statistically homogeneous and comparable. There was no case of urgency in this study. Three children from the aprotinin group were excluded from the study due to detection of pre-operative infection (one case), and use of corticosteroids by indication of anesthesia (two cases, one from each group) after randomization.
Intraoperative
In the Aprotinin and Control groups, the length of surgery and anesthesia, time of CPB and aortic clamping (anoxia) and oropharyngeal minimum temperature (hypothermia) in CPB showed no statistically significant difference. Oxygenators had a similar distribution in both groups. The total volume of perfusate and additions were slightly higher in the control group, but with no significant difference. The estimated blood volume and the use of red blood cells during CPB were similar in both groups. As the blood balance intraoperatively, it tended to be negative in the aprotinin group (P=0.05)(Table 2).
The total amount of aprotinin administered was 177.56±77.76mg, corresponding to 126.84±55.55ml of Trasylol. The total amount of heparin was similar (39.2±15.3mg, median = 33.5mg vs. 39.0±16.7 mg, median = 30mg), corresponding to 7.4±2.9 vs. 7.1±3.8mg/kg (P>0.05).
The hemofiltrated volume (378±244ml vs. 335±319ml) in CPB and diuresis (ml/kg) of both groups before (4.60±4.39 vs. 2.64±2.64), during (19.22±18.53 vs. 18.13±17.81) and after (21.22±31.64 vs. 11.93±5.66) CPB was similar (P>0.05). The time of closure of the chest was higher (28.5±9.1 min vs. 28.8±17.4 min).
Postoperative
The water balance at admission to the PICU was similar in both groups (24±77ml/kg, median = 25ml/kg vs. 31±32ml/kg, median = 35ml/kg)(P=0.60). The PRISM score tended to worse in the control group, but without significant difference (Table 2).
The two groups were similar regarding clinical-surgical index by Mattos (5.3±2.2, median = 5.5 vs. 4.7±1.6, median = 5)(
P> 0.05), confirming that groups are suitably comparable and homogeneous (Table 3). Seventy percent of children of aprotinin group and 88.8% of the control group were in the intermediate risk category (ages between 1 month and 1 year). Protein-energy malnutrition occurred in 90% vs. 77.7% of children, and almost all with high-risk criterion (below the 5th percentile). By the presence of heart failure, pulmonary hypertension and/or genetic syndrome (associated clinical risk factors), nearly half (50% vs. 55.5%) of children of both groups were high risk. All children in the control group were suitable for the category of low risk as for surgical complexity (Aristotle basic score), occurring in 80% of the aprotinin group (due to intraoperative diagnosis of DORV)(Table 3). As for the length of CPB, all patients - except for one group of aprotinin that exceeded 90 minutes - were at intermediate risk.
There was no statistically significant differences between groups in times of inhaled nitric oxide (a case in aprotinin group and two cases in the control)(216 vs.118 h), mechanical ventilation, stay in the PICU and use of inotropes (Table 2).
In the Aprotinin and Control groups, we found severe pulmonary congestion (four vs. three patients), circulatory shock (five vs. three patients), anasarca in four (two with ascites) vs. two patients and pleural effusions (two in each group). Both groups showed hyperthermia (37.8±1.2ºC vs. 37.07±1.1ºC) 4h after administration of protamine (T5)(
P=0.09), practically, and normalizing in T7 and T8. The PaO
2/FiO
2 relationship was similar in both groups. The drop in T7 in relation to T5 (246.38±129.39 vs. 224.14±165.71) was significant only in the control group (
P=0.04).
Bleeding in the first 48 h postoperatively was similar in both groups (17.6 vs. 18.1ml/kg)(
P>0.05). One patient in the aprotinin group was transfused with packed red blood cells (10ml/kg) in T5, due to anemia, according to the protocol of PICU. Platelets (12ml/kg) was used in two patients in the control group (T6 and T7, respectively). The number of donor blood products that children were exposed to from both groups was similar (median 2).
Diuresis until T5 (4h after protamine) was similar in both groups (19.7±7.8 vs. 22.1±22.3ml/kg)(P=0.39), followed by the slight decrease at T6 (11.2±5.6 vs. 14.7±5.6ml/kg)(P=0.16), with subsequent significant increase in both groups, T8 (43.0±13.8 vs. 69.7±32.4)(
P=0.19).
In T5, there was great similarity between groups as for uremia (18.0±5.2 vs. 17.4±6.9g/dl) and serum creatinine (0.3±0.1 vs. ± 0.3 0.1mg/dl), which increased significantly from T5 to T8, when uremia corresponded to 37.2±11.5 vs. 28.0±11.4g/dl (
P<0.01), and plasma creatinine, 0.56±0.15 (
P<0.01) vs. 0.48±0.22mg/dl (
P=0.02). There was no difference between groups (
P>0.13).
The rates of hemoglobin (9.6±1.1 vs. 8.9±1.3)(
P=0.40) and hematocrit (29.3±2.8 vs. 27.5±4.2%)(
P=0.49) levels were slightly lower at T1 in the control group, but with no significant difference. Both had a significant drop from T1 to T2 (7.6±1.9 vs. 9.59±1.5g/dl and 23.4±5.6 vs. 29.5±3.9%)(
P<0.01), succeeded by progressive hemoconcentration up to T5 (12.1±1.9 vs. 12.7±2.9g/dl and 37.6±4.9 vs. 40.2±8.6%), whose value was statistically higher than T1 (
P<0.01). In T2 and T3, the gemoglobina and hematocrit were significantly lower in the aprotinin group compared to control group (
P<0.01).
The WBC count (no. cells/mm
3), similar in both groups (7580±2948 vs. 6711±1835)(
P=0.62) in T1, decreased with the onset of CPB (T2)(Figure 1). This nadir in T2 reached statistical intragroup significance in Aprotinin group (3722±1115 vs. 5411±2599)(
P<0.01), with intergroup difference also statistically significant (
P=0.04). Before the disconnection of CPB (T3), the leukocyte counts tended to recover only in the control group (7675±2961) compared to T1 (
P=0.40), whereas in the aprotinin group remained just below T1 (6422±3574)(
P=0.19)(Figure 1). After CPB, leukocytosis was significant from T4 to T8 in both groups (
P<0.01), with a peak in T7 (15 866±8991 vs. 16 680±4646.72). There was no significant intergroup difference from T3 to T8 (
P>0.33).
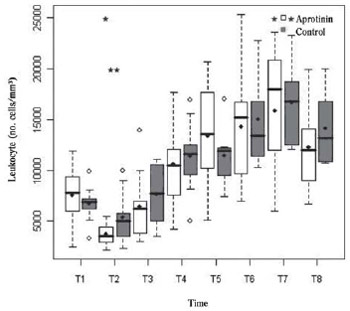
Fig. 1 - Leukocyte count (no./mm
3) in the Aprotinin and Control groups, at times T1 to T8. Intragroup (*) and intergroups (**) differences statistically significant are indicated (P-value with Box-Cox transformation)
With regard to inflammatory markers in T1, basal TNF-á was detectable in both groups, with no significant difference (
P=0.10). The basal levels of IL-6 and IL-10 in both groups were supranormal and similar (
P=0.10). The largest proportion IL-6/IL-10 Aprotinin group did not reach statistical significance in relation to Control (19.14±31.98, median = 10.55 vs. 5.72±7.59, median = 2.36)(
P=0.54).
The kinetic profile of cytokines (Table 4) was not affected by aprotinin, being similar between groups, and expressed transient elevation of TNF-α (Figure 2) of biphasic pattern with peak at T2, significant only in the Control group (
P<0.01), and peaks from T4 to T5, with no significant difference in relation to T1 (
P=0.25). Prolonged increase of IL-6 (Figure 3), T4 to T7 (
P<0.01), and the chemokine IL-8 (Figure 4) with peak at T4 in both groups (
P<0.01) was performed with no difference between groups. There was an early release of IL-10 (Figure 5), with maximum serum concentration after reperfusion in T4 in both groups (
P<0.01). In T3 (before disconnection of CPB), the IL-10 of the Control group was higher (P=0.02). There were no significant correlations between these variables.

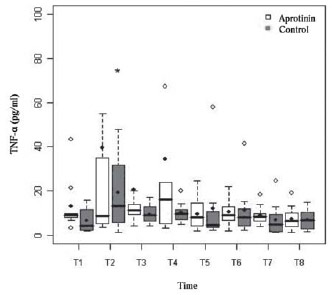
Fig. 2 - Biphasic serum profile of serum concentration of Tumor Necrosis Factor-alpha (TNF-á) in pg/ml in Aprotinin and Control groups, the times from T1 to T8. Statistically significant intragroup differences are indicated (*) (P-value with Box-Cox transformation)

Fig. 3 - Serum levels of IL-6 (pg/ml) in the Aprotinin and Control groups, the times T1 to T8. The intragroup differences statistically significant are indicated (*) (P-value with Box-Cox transformation)
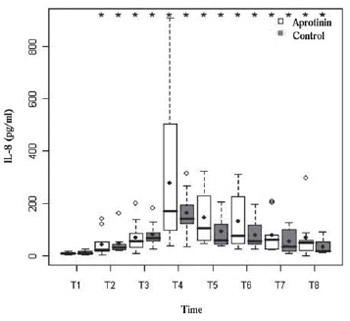
Fig. 4 - Serum levels of IL-8 (pg/ml) in the Aprotinin and Control groups, the times T1 to T8. Statistically significant intragroup differences are indicated (*) (P-value with Box-Cox transformation)
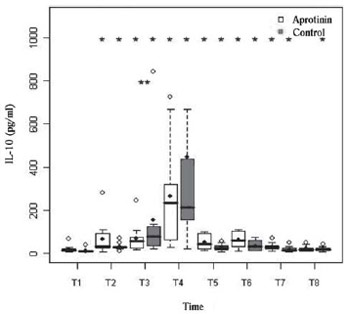
Fig. 5 - Serum levels of IL-10 (pg/ml) in the Aprotinin and Control groups, the times T1 to T8. The differences were statistically significant, intra- (*) (P-value with Box-Cox transformation) and intergroup (**) (P-value with Box-Cox transformation) are indicated
CPB induced decrease in IL-6/IL-10 ratio in both groups (Figure 6). In both groups there were two peaks of this fraction, one in T5 and another, higher, in T7 (median = 13.66±14.08 vs 6.88. 20.58±16.74 median = 15.70), and in T8, remaining in the Aprotinin group below the baseline (10.59±14.07 median = 6.40)(P=0.25) and Control group, tending above the baseline (12.10±11.43 median = 5.28)(P=0.06). In the Control group, both peaks were significantly higher than T1 (P<0.01). There was no difference between groups.

Fig. 6 - IL-6/IL10 proportion in the Aprotinin and Control groups, the times T1 to T8. Statistically significant intragroup differences are marked (*) (P-value with Box-Cox transformation)
As previously established criteria, SIRS was observed in 30% of cases of Aprotinin group and in 44.4% of the Control group. The SCLS occured, respectively, in 40% and 22.2% of them. Patients who developed SIRS in both groups had greater clinical modified Ross score, 7 to 8 and 2 to 8 respectively, while in others (without SIRS), these scores were 0 to 7 and 0 to 6. We also note that children with preoperative Ross scores greater than or equal to 5, regardless of group (Aprotinin or Control), had longer durations of mechanical ventilation, stay in the PICU and hospital stay, compared to children with scores less than 5 (P<0.05).
The clinical-surgical index by Mattos, in patients with SIRS and/or SCLS, had a median of 6 and the others from 4.5 to 5.
In both groups, there were cardiovascular disorders (60% vs. 33.3%), renal (30% vs. 22.2%) and respiratory (40% vs. 22.2%), with no statistically significant difference. Two children in the Cntrol group experienced hematologic dysfunction. No cases of neurological or hepatic dysfunction. Infectious pulmonary complications occurred in both groups (20% vs. 33.3%).
The time of stay in PICU and hospital stay were not significantly altered by SIRS and/or SCLS. There were no deaths. All patients were discharged from hospital in good clinical and healing condition. The postoperative echodopplercardiography evaluation demonstrated good outcome in all patients. There were no allergic problems, hypotension, nor thrombosis, as adverse effects of aprotinin.
DISCUSSION
It was discussed a lot aprotinin usage in cardiac surgery for hemostatic control as for the cost-effectiveness, the high cost and possible adverse effects that contraindicate its use in cases of low risk. However, there was potential usefulness in children predisposed to bleeding and systemic inflammatory syndrome [5,16], as those who has undergone surgery in Brazil, with a significant degree of malnutrition. In order temporarily suspending the manufacture of aprotinin, although in children it has been not reported significant adverse effects as noted in adults [11-13], and associated with the fact that there is little information available about its influence on the systemic inflammatory response at hemostatic doses, these considerations have led to this study, which aimed to show hemostatic benefit with the use of aprotinin in high doses in acyanotic cardiopathic children operated with CPB by assessing the possible anti-inflammatory effect associated, which was not confirmed in this series.
The age range in this study was restricted to the period from 30 days to 4 years of life in acyanotic children in order to try to reduce the spread of age and type of heart disease, in contrast to similar studies, because they approached cyanotic children, acyanotic and neonates with various heart diseases in complexity.
We noted that in our hospital, the occurrence of acyanogenic congenital heart disease in this age group chosen is not very frequent, being reported about 30 cases per year on average (unpublished data, from 2005 and 2006). The 19 patients included in this study were considered, therefore, a representative sample of the population of individuals who present this heart disease and undergo surgery in our hospital, because such population is not large. The newborns were excluded due to their greater propensity to complications of CPB [15] secondary to the intense neuroendocrine response to tissue injury, the large release of cytokines and lower neutrophil response [17] compared to children older than 1 month old.
Despite the estimated in-hospital or 30-day mortality, from 1 to 5%, based on risk scores more widespread (RACHS-1 and Aristotle), no deaths occurred in this study. The good surgical outcome was reinforced by the clinical-surgical index, more recently, proposed in Brazil [15], whose scores (5.5 vs. 5) correspond to risks of death from 11.70% to 23.98%, due mainly to the prevalence of low body weight, indicative of malnutrition, and genetic syndromes, pulmonary hypertension and congestive heart failure, which certainly influenced the morbidity. These similar indices and scores of the operated groups confirm the homogeneity needed for analysis, highlighting the variability in the weights, which are incompatible with regard to age, especially due to the nutritional changes resulting from heart diseases.
Since it is believed that aprotinin reduces bleeding and the need for transfusion through dose-dependent mechanism [6], we opted to use the pioneer protocol (Hammersmith Hospital), called high dose, proportional to body surface, in order to optimize the clinical benefit, since higher doses might be required for the anti-inflammatory action [18]. With calculations based on body surface area, children receive higher doses of aprotinin than when the doses are based only on weight. Unfortunately, the dosage, of course, had the side effect of introducing a bias of greater hemodilution in the treated group, as confirmed by significantly lower levels of hemoglobin and hematocrit in CPB.
Within the reality of our hospital, commercial limitations led to the inclusion of two main models of oxygenators, a potentially serious failure due to the possible difference in biomaterial-dependent responses in the CPB blood-circuit interactions. However, it is one aspect still controversial currently, and it is reasonable to assume that this has not occurred, because in addition to the distribution of oxygenators have been similar in both groups, Dubois et al. [19] in 2004, demonstrated performance and degree of hemolysis similar of two (Lilliput and Safe Micro) of the most used in this study. In addition, Jensen et al. [20] in 2003, found that a hollow fiber membrane oxygenator used here, act similarly in both coagulation and fibrinolysis systems, as in serum cytokines concentrations TNF-α, IL-6 and IL-8, which proved to be even similar when other oxygenators coated with heparin were used.
In the Aprotinin group, the WBC count was significantly lower than in the Control group and remained below baseline in the CPB. This may have resulted from the action of the drug that is known to be effective, even at low doses, in reducing the mobilization of neutrophils at the end of CPB [18]. However, aprotinin did not affect postoperative leukocytosis, with peak of 24 hours after protaminization and recognized as part of systemic inflammatory response [2,18].
As expected [21], circulating pro-inflammatory cytokines, undetectable in healthy children, were high in preoperative samples of this investigation and the same goes for the IL6/IL-10 relationship. As we excluded the cases with clinical, laboratory and radiographic evidences of infection or inflammation, this finding suggests the presence of subclinical inflammatory condition [22,23] generated by chronic severe hemodynamic dysfunction, inducing myocardial synthesis of cytokines, exacerbated by feedback by their systemic levels [24]. The preoperative detection of TNF-α, usually related to endotoxemia [3], whose increase, in general, precedes that of other cytokines [22], may have been influenced by anesthesia [25]. Along with some additional release during CPB, TNF-α may have triggered the cascade of cytokine-dependent clinical postoperative manifestations [3], consisting of fever, hypotension, SCLS, SIRS, coagulopathy and renal dysfunction. We also know that the use of RBC in the perfusate can also provide exogenous cytokines and cause and/or contribute to the increase in IL-6, both during CPB and postoperative [3].
The kinetic profile of cytokines was not affected by aprotinin. In both groups, it expressed typical transient elevation of biphasic pattern, with early peaks of TNF-α at the start of CPB and 4 h after protaminization [17], preceding the prolonged increase in IL-6 and increased chemokine IL-8, a regulator of leucodiapedesis to sites of inflammation [3].
Although aprotinin, such as prednisolone, can inhibit production of TNF-α during CPB [3], this does not occur in this study, similar to other studies in adults [26]. In similar research performed in our Service by Carmona [27] in 52 operated children, all under treatment with aprotinin, the kinetic profile of TNF-α was similar to this study. The IL-6 is a marker of cellular injury with pyrogenic and inflammatory prolonged effect in children undergoing CPB [17], although, interestingly, it also has anti-inflammatory activity. The fact of Aprotinin did not have attenuated the release of this cytokine agrees with the only other two previous randomized studies - performed in children [6.18] - and with an observational study by Carmona [27]. The IL-8 produced by macrophages, although with a pathophysiological role considered limited and controversial, has potent recruiter action of leukocytes to inflammatory foci, particularly pulmonary foci, in addition to mediating SIRS and myocardial dysfunction [24,27].
We observed progressive and similar intergroup increase of IL-8, coinciding with the IL-6, succeeding rewarming and aortic unclamping, with a peak of 5 minutes after protaminization. This finding was similar to other studies in children [17.27]. This is also consistent with studies of Mössinger et al. [6] in 2003 and Carmona [27] in 2006, both in children and with others in adults [26], in which there was no decrease in IL-8 with aprotinin, in spite of recent reports on adults and atributing to the aprotinin an effect comparable to that of corticosteroids, as regards the reduction of IL-8 [28].
The IL-10, whose production is stimulated by TNF-á inhibits the synthesis of cytokines TNF-á, IL-1b and IL-6, induces down regulation of the production of IL-8 and inactivates macrophages and monocytes [29]. In our study, we observed a significant increase in IL-10 since the start of CPB, with peak after 5 min of protamine infusion, a pattern consistent with previous studies in children [27,30,31] and a behavior similar to that found in adults [29]. Despite the disagreement with studies in adults [4], increased serum IL-10 was not affected by aprotinin, a finding similar to other studies in children [6.27]. Nevertheless, there were lower levels of IL-10 shortly before the end of CPB, with the use of aprotinin. In a recent meta-analysis of studies on the antiinflammatory effect of aprotinin in adults, there was a reduction of IL-10, but it occured soon after protamine administration and under use of low-dose aprotinin [32]. In this meta-analysis it was not found significant effects of aprotinin in high doses on all other markers of systemic cytokines assessed in our study.
According to Taniguchi et al. [33] in 1999, in patients who died of septic shock and multiple organ failure, concentrations of IL-6 increased gradually as it occurs down regulation of IL-10, making the proportion IL-6/IL-10 increases. Therefore, the IL-6/IL-10 proportion is a recognized predictor of poor outcome in sepsis. In this study, this fraction increased from 4h after protamine administration, with peak at 24h in both groups but was significant only in the Control group. This finding, suggesting a beneficial effect of aprotinin, is of difficult discussion when considering the lack of previous observations in studies similar to this.
It was not also observed antiinflammatory clinical efficacy of aprotinin in our study, occurring SIRS and SCLS in both groups, with a tendency more marked in children with worse preoperative clinical presentation of CHF (higher modified Ross scores), also reflecting on the postoperative with longer durations of mechanical ventilation, stay in the PICU and hospital stay when the score was greater than or equal to 5, suggesting the importance of subclinical proinflammatory condition before surgery [22,23]. There was also more tendency for the occurrence of SCLS in the aprotinin group.
The significant decrease in postoperative PaO2/FIO2 relationship in the control group, consistent with acute lung injury was not significant in the Aprotinin group, and we may infer anti-inflammatory protective effect of Aprotinin, although clinically ineffective, consistent with the recent report by Williams et al. [34] in 2008, and contrary to the report by Mössinger et al. [6] in 2003, who observed reduction in the duration of mechanical ventilation with the use of drugs.
The PRISM score at PICU admission (3 vs. 7) Suggested that the drug improved the initial hemodynamic and metabolic stability in Aprotinin group, but the clinical postoperative evolution of the groups was similar. In the Aprotinin group, there was practically a higher incidence of organ dysfunction, both cardiovascular (60% vs. 33.3%) and respiratory (40% vs. 22.2%), although without statistically significance. These rates, however, were within the expected range for age, because it is accepted that 50% of patients present pulmonary and cardiovascular disorders [2]. There were similarity for total postoperative bleeding between groups, below the reported in the literature [6], but the hematologic dysfunction (thrombocytopenia with bleeding) occurred in the Control group.
There was no intergroup difference regarding the incidence of postoperative infection. Although in some clinical investigations with Aprotinin it is argued that the drug increases the risk of mediastinitis [35], at the same time there are other groups who use it routinely in heart and lung transplants, without increasing the rate of infection [36].
One limitation of our study is the small number of cases for comparison between groups, collected from a single center, although prospective and randomized, and the data obtained in a given period, with procedures being performed by the same surgical, anesthetic, perfusion and postoperative teams. The groups were homogeneous and comparable (diagnostics, surgeries and modified Ross, RACHS-1, Aristotle basic, and Mattos scores), characterizing a representative sample within the reality of occurrence of the population and age range of acyanotic congenital heart diseases patients selected who had undergone cardiac surgery at our hospital, excluding newborns, as mentioned at the beginning of this discussion.
On the other hand, we used the most powerful test for the sample compared with the non-parametric test, and the statistical analysis by means of this model (multiple regression), with fixed effects (group and time fixed) and random (particularity of each child), was performed to assess the residue, by checking whether the distribution is normal. With the assumptions of residual normality and constant variance (similar variability comparing the two groups) met, it was allowed to demonstrate that the model and the sample were viable for parametric statistical analysis, providing results in accordance with the assumptions of the model, including with transformations of logarithmic type or Box-Cox. The risk is that a possible significance between the groups - that might exist if there was a larger sample - cannot be detected, as in bleeding or in serum levels of cytokines. The possible error is that our study sample is part of the sample of the 5% that do not represent the population (type I error). The certainty of success is 95% (confidence interval).
Thus, in this series, the high hemostatic dose of aprotinin did not provide significant changes in serum inflammatory markers and did not influence clinical manifestations of systemic inflammatory syndrome and capillary leak, but there was a greater cardiovascular and respiratory dysfunction in the group that it was used. Reproduction of this study with broadening of sampling is therefore desirable and necessary in multicenter studies and/or services and surgical teams with the highest number of children operated on this type of heart disease, but it is currently hampered by the removal of aprotinin from the market.
CONCLUSIONS
In this series, high hemostatic dose of aprotinin did not minimize postoperative clinical manifestations and serum markers of systemic inflammatory response.
1. Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250-6. [MedLine]
2. Seghaye MC. The clinical implications of the systemic inflammatory reaction related to cardiac operations in children. Cardiol Young. 2003;13(3):228-39. [MedLine]
3. Laffey JG, Boylan JF, Cheng DC. The systemic inflamatory response to cardiac surgery: implications for the anesthesiologist. Anesthesiology. 2002;97(1):215-52. [MedLine]
4. Wegner J. Biochemistry of serine protease inhibitors and their mechanisms of action: a review. J Extra Corpor Technol. 2003;35(4):326-38. [MedLine]
5. Oliver WC Jr, Fass DN, Nuttall GA, Dearani JA, Schrader LM, Schroeder DR, et al. Variability of plasma aprotinin concentrations in pediatric patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2004; 127(6): 1670-7. [MedLine]
6. Mössinger H, Dietrich W, Braun SL, Jochum M, Meisner H, Richter JA. High-dose aprotinin reduces activation of hemostasis, allogeneic blood requirement, and duration of postoperative ventilation in pediatric cardiac surgery. Ann Thorac Surg. 2003;75(2):430-7. [MedLine]
7. Tweddell JS, Hoffman GM, Mussatto KA, Fedderly RT, Berger S, Jaquiss RD, et al. Improved survival of patients undergoing palliation of hypoplastic left heart syndrome: lessons learned from 115 consecutive patients. Circulation. 2002;106(12 Suppl 1):I82-9. [MedLine]
8. Wippermann CF, Schmid FX, Eberle B, Huth RG, Kampmann C, Schranz D, et al. Reduced inotropic support after aprotinin therapy during pediatric cardiac operations. Ann Thorac Surg. 1999;67(1):173-6. [MedLine]
9. Fergusson DA, Hébert PC, Mazer CD, Fremes S, MacAdams C, Murkin JM, et al. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med. 2008;358(22):2319-31. [MedLine]
10. Twite MD, Hammer GB. The use of aprotinin in pediatric cardiac surgery: should we bid 'good riddance' or are we throwing out the baby with the bath water? Paediatr Anesth. 2008;18(9):809-11.
11. Backer CL, Kelle AM, Stewart RD, Suresh SC, Ali FN, Cohn RA, et al. Aprotinin is safe in pediatric patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2007;134(6):1421-6.
12. Guzzetta NA, Evans FM, Rosenberg ES, Fazlollah TM, Baker MJ, Wilson EC, et al. The impact of aprotinin on postoperative renal dysfunction in neonates undergoing cardiopulmonary bypass: a retrospective analysis. Anesth Analg. 2009;108(2):448-55. [MedLine]
13. Manrique A, Jooste EH, Kuch BA, Lichtenstein SE, Morell V, Munoz R, et al. The association of renal dysfunction and the use of aprotinin in patients undergoing congenital cardiac surgery requiring cardiopulmonary bypass. Anesth Analg. 2009;109(1):45-52. [MedLine]
14. Goldstein B, Giroir B, Randolph A; International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2-8. [MedLine]
15. Mattos SS, Neves JR, Costa MC, Hatem TP, Luna CF. An index for evaluating results in paediatric cardiac intensive care. Cardiol Young. 2006;16(4):369-77. [MedLine]
16. Arnold DM, Fergusson DA, Chan AK, Cook RJ, Fraser GA, Lim W, et al. Avoiding transfusions in children undergoing cardiac surgery: a meta-analysis of randomized trials os aprotinin. Anesth Analg. 2006;102(3):731-7. [MedLine]
17. Alcaraz AJ, Manzano L, Sancho L, Vigil MD, Esquivel F, Maroto E, et al. Different proinflammatory cytokine serum pattern in neonate patients undergoing open heart surgery. Relevance of IL-8. J Clin Immunol. 2005;25(3):238-45. [MedLine]
18. Seghaye MC, Duchateau J, Grabitz RG, Jablonka K, Wenzl T, Marcus C, et al. Influence of low-dose aprotinin on the inflammatory reaction due to cardiopulmonary bypass in children. Ann Thorac Surg. 1996;61(4):1205-11. [MedLine]
19. Dubois J, Jamaer L, Mees U, Pauwels J, Briers F, Lehaen J, et al. Ex vivo evaluation of a new neonatal/infant oxygenator: comparison of the Terumo CAPIOX Baby RX with Dideco Lilliput 1 and Polystan Safe Micro in the piglet model. Perfusion. 2004:19(5):315-21. [MedLine]
20. Jensen E, Andréasson S, Bengtsson A, Berggren H, Ekroth R, Lindholm L, et al. Influence of two different perfusion systems on inflammatory response in pediatric heart surgery. Ann Thorac Surg. 2003;75(3):919-25. [MedLine]
21. Chew MS, Brandslund I, Brix-Christensen V, Ravn HB, Hjortdal VE, Pedersen J, et al. Tissue injury and the inflammatory response to pediatric cardiac surgery with cardiopulmonary bypass: a descriptive study. Anesthesiology. 2001;94(5):745-53.
22. Mou SS, Haudek SB, Lequier L, Peña O, Leonard S, Nikaidoh H, et al. Myocardial inflammatory activation in children with congenital heart disease. Crit Care Med. 2002; 30(4):827-32. [MedLine]
23. Hövels-Gurich HH, Schumacher K, Vazquez-Jimenez JF, Qing M, Huffmeier U, Buding B, et al. Cytokine balance in infants undergoing cardiac operation. Ann Thorac Surg. 2002;73(2):601-8.
24. Hövels-Gurich HH, Vazquez-Jimenez JF, Silvestri A, Schumacher K, Minkenberg R, Duchateau J, et al. Production of proinflammatory cytokines and myocardial dysfunction after arterial switch operation in neonates with transposition of the great arteries. J Thorac Cardiovasc Surg. 2002;124(4):811-20. [MedLine]
25. Brancaccio G, Villa E, Girolami E, Michielon G, Feltri C, Mazzera E, et al. Inflammatory cytokines in pediatric cardiac surgery and variable effect of the hemofiltration process. Perfusion. 2005;20(5):263-8. [MedLine]
26. Schmartz D, Tabardel Y, Preiser JC, Barvais L, d'Hollander A, Duchateau J, et al. Does aprotinin influence the inflammatory response to cardiopulmonary bypass in patients? J Thorac Cardiovasc Surg. 2003;125(1):184-90. [MedLine]
27. Carmona F.Avaliação dos fatores de risco para disfunção miocárdica e mortalidade intra-hospitalar em neonatos e lactentes submetidos a cirurgia cardíaca com circulação extracorpórea [Dissertação de Mestrado]. Ribeirão Preto: Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo;2006. 153p.
28. Greilich PE, Brouse CF, Whitten CW, Chi L, Dimaio JM, Jessen ME. Antifibrinolytic therapy during cardiopulmonary bypass reduces proinflammatory cytokine levels: a randomized, double-blind, placebo-controlled study of epsilonaminocaproic acid and aprotinin. J Thorac Cardiovasc Surg. 2003;126(5):1498-503. [MedLine]
29. Caputo M, Bays S, Rogers CA, Pawade A, Parry AJ, Suleiman S, et al. Randomized comparison between normothermic and hypothermic cardiopulmonary bypass in pediatric open-heart surgery. Ann Thorac Surg. 2005;80(3):982-8. [MedLine]
30. Sugita T, Watarida S, Katsuyama K, Nakajima Y, Yamamoto R, Mori A. Interleukin-10 concentration in children undergoing cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1996;112(4):1127-8. [MedLine]
31. Tárnok A, Hambsch J, Schneider P. Cardiopulmonary bypassinduced increase of serum interleukin-10 levels in children. J Thorac Cardiovasc Surg. 1998;115(2):475-7. [MedLine]
32. Brown JR, Toler AW, Kramer RS, Landis RC. Antiinflammatory effect of aprotinin: a meta-analysis. J Extra Corpor Technol. 2009;41(2):79-86. [MedLine]
33. Taniguchi T, Koido Y, Aiboshi J, Yamashita T, Suzaki S, Kurokawa A. Change in the ratio of interleukin-6 to interleukin10 predicts a poor outcome in patients with systemic inflammatory response syndrome. Crit Care Med. 1999;27(7):1262-4. [MedLine]
34. Williams GD, Ramamoorthy C, Pentcheva K, Boltz MG, Kamra K, Reddy VM. A randomized, controlled trial of aprotinin in neonates undergoing open-heart surgery. Pediatr Anesth. 2008;18(9):812-9.
35. Carrel TP, Schwanda M, Vogt PR, Turina MI. Aprotinin in pediatric cardiac operations: a benefit in complex malformations and with high-dose regimen only. Ann Thorac Surg. 1998;66(1):153-8. [MedLine]
36. Jaquiss RD, Huddleston CB, Spray TL. Use of aprotinin in pediatric lung transplantation. J Heart Lung Transplant. 1995;14(2):302-7. [MedLine]
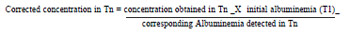










 All scientific articles published at www.rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at www.rbccv.org.br are licensed under a Creative Commons license