Shi-Min YuanI
ABSTRACT
Aberrant origin of vertebral artery is rare. The anatomical features and clinical significance of this lesion remain to be clarified. A comprehensive collection of the pertinent literature resulted in a cohort of 1286 cases involving 955 patients and 331 cadavers. There were more left than right and more unilateral than bilateral aberrant vertebral arteries. Patients with aberrant origin of vertebral artery were often asymptomatic and in only 5.5% of the patients their symptoms were probably related to the aberrant origin of vertebral artery. The acquired cardiovascular lesions were present in 9.5% of the patients, 20.9% of which were vertebral artery-associated lesions. Eight (0.8%) patients had a vertebral artery dissection. Logistic regression analysis showed significant regressions between bovine trunk and left vertebral artery (P=0.000), between the dual origins of vertebral artery and cerebral infarct/thrombus (P=0.041), between associated alternative congenital vascular variants and cervical/aortic dissection/atherosclerosis (P=0.008). Multiple logistic regression demonstrated that side of the aberrant origin of vertebral artery (left vertebral artery) (P=0.014), arch branch pattern (direct arch origin) (P=0.019), presence of the common trunk (P=0.019), associated acquired vascular disorder (P=0.034) and the patients who warranted management (P=0.000) were significant risk predictors for neurological sequelea. The patients with neurological symptoms and those for neck and chest operations/interventions should be carefully screened for the possibility of an aberrant origin of vertebral artery. The results from the cadaver metrology study are very helpful in the design of the aortic stent. The arch branch pattern has to be taken into consideration before any maneuver in the local region so as to avoid unexpected events in relation to aberrant vertebral artery.
QUOROM = Quality of Reporting of Meta-Analyses
VA = Vertebral artery
INTRODUCTION
The vertebral artery (VA), which usually arises from the posterosuperior aspect of the first part of the subclavian artery and enters into the intracranial space via the dura mater at first cervical vertebra (C) and reaches C6 after traveling through the foramen transversarium, is an important blood supply of the brainstem and cerebellum[1]. VA pathologies, including anomalous origin and course, dual arteries, duplication, fenestration, tortuosity, elongation, kinking, arachnoid cysts, aneurysmal formation and associated hereditary connective tissue disorder, implicate typically in cerebrovascular events as a source of blood supply of posterior circulation[2]. Steal syndromes can be present in the condition of certain situations characterized by VA flow inversion[3]. Aberrant origin of VA is a rare variant of VA pathologies implicating in not only cerebrovascular events, but also VA dissection[4] and surgical anatomy of local regions in particular in the operation of the carotid artery[5] or aortic arch[6]. The "vertebral arteria lusoria", although even more rarely seen, should be considered in the patients undergoing esophageal surgery, and unawareness of such an aberrant VA may cause life-threatening events[7]. Moreover, dual origins of VA should be recognized in preoperative evaluation of patients with extracranial vascular disease[8]. This article aims to highlight the clinical importance and surgical anatomy of the aberrant origin of VA.
METHODS
Publications in English language reporting on aberrant VA until February 2015 were retrieved in MEDLINE, Highwire Press and Google search engines. The search terms "aberrant origin", "dual/duplicated/bifid origins", "vertebral artery" and "aortic arch branching" were searched.
Primary exclusion criteria included articles describing aberrant origin of VA without giving patient number, other types of lesions of VA than aberrant origin. Studies with no complete data were excluded for the pertinent statistical analyses. Data were carefully extracted for details of the patient population, demographics, clinical symptoms, characteristics of VA, aortic branching pattern, common trunk of the artery, entry of VA into the foramen transversarium, associated congenital/acquired vascular disorders, associated otherwise disorders and cerebral events, etc. This rare condition was mostly reported in sporadic single cases or small series while a few with larger patient population. Accordingly, the qualitative analysis of the collective data from the retrieved articles constituted a systematic review, as suggested in the Quality of Reporting of Meta-Analyses (QUOROM) recommendations.
The study subjects were divided into two groups, cadaver and patient groups. The anatomy study involved both groups; while the clinical study was performed solely in the patient group.
Quantitative data were presented as mean ± standard deviation along with range and median values, and intergroup differences were compared by unpaired t-test. Comparisons of frequencies were performed by Fisher's exact test. P<0.05 was considered statistically significant.
RESULTS
The literature retrieval generated a total of 214 articles with 1286 cases involving 955 (74.3%) patients and 331 (25.7%) cadavers.
There were 345 (58.5%) males and 245 (41.5%) females of the studying subjects whose gender was given. The gender ratio was 1.41. The patients' age was 48.2±21.0 (range, 0-89; median, 51) years (n=130) and the cadaver's' age was 57.4±23.4 (range, 0-94; median, 61) years (n=46).
The presenting symptoms or causes for presentation were described in 168 (17.6%) individuals of the patient group. Five (3.0%) patients were asymptomatic and 163 (97.0%) were symptomatic. Of the latter, the symptoms were associated probably with the lesions of the aberrant VA in 9 (5.5%) (Table 1), and due to lesions other than aberrant VA (including cerebral hemorrhage, cervical arterial disorder, post-traumatic syndrome, or aortic dissection) in the remaining 154 (94.5%) patients.
There were more left than right and more unilateral than bilateral aberrant VAs (Table 2).

The single aberrant origin and dual origins of VAs were depicted in Tables 3 and 4. Hypoplastic VAs were found in 28 (2.2%) cases. Abnormal course of the aortic arch branches was found in 31 (2.4%) cases, with aberrant right VA being the most common (60%). The fusion level of the dual origins of the VAs was reported in 20 (1.6%) patients for 21 pairs of VAs, of which the left VAs fused most frequently at C5-6 (33.3%). Level of entry of the VAs into the foramen transversarium were expressed for 100 left and 33 right VAs with most of the left VAs entering into C5 (43%) and most of the right entering into C7 (21.2%).
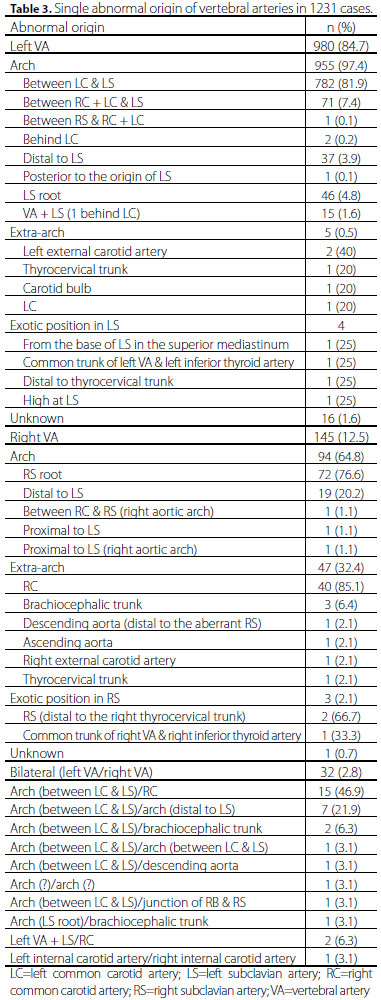

Aortic arch branching was stated in 1270 (98.7%) cases, including 1 (0.1%) 2-vessel, 236 (18.6%) 3-vessel, 1011 (79.7%) 4-vessel and 21 (1.7%) 5-vessel aortic arch branching patterns. The brachiocephalic trunk-left common carotid artery-left VA-left subclavian artery pattern was the most common 4-vessel arch branching pattern accounting for 84.8% (Figure 1). Two hundred and eleven common trunks of the arteries were found in 207 (16.1%) cases with one common trunk in 203 (98.1%) patients and two common trunks in 4 (1.9%). The bovine trunk (i.e., the common trunk of the brachiocephalic trunk and left common carotid artery) was the most frequent arterial trunk seen in this cohort.

A total of 115 (12.0%) patients had one or more congenital cardiovascular anomalies, where aberrant right subclavian artery was the most common (55.7%). In the patient group, 89 (9.3%) patients had one or more acquired vascular associations, including aneurysms, obstructions, stenosis, thrombus formation and dissection, etc., 18 (20.2%) of which were VA-associated lesions and 8 (0.8%) having a VA dissection.
There were 43 (4.5%) patients with cerebrovascular lesions, including cerebral infarcts[9-24] in 17 (39.5%), cerebral vascular aneurysms without cerebral hemorrhage[14,23,25,26] in 4 (9.3%) and cerebral hemorrhage in 18 (41.9%), and ischemic cerebrovascular disease[25], transient ischemic attack[14], partial thrombus detected in the left internal jugular vein and sigmoid sinus[27], and vertebrobasilar insufficiency[28] in 1 (2.3%) patient each. The 18 cerebral hemorrhages were subarachnoid hemorrhage in 14 (77.8%)[29-39] (8 were associated with an aneurysm of the cerebral artery[29-31,33,35-37,39]), intraventricular hemorrhage in 2 (11.1%)[40,41] and intracerebral hemorrhage[42] and subarachnoid and intraventricular hemorrhages[43] in 1 (5.6%) patient each.
The cardiovascular, cerebrovascular, or orthopedic complications, accounting for 84.8% (78/92), 13.7% (13/95) and 4.2% (4/95), respectively, were described along with their management in 95 (9.9%) patients. There were no significant differences in the curative ratio and mortality between the cardiovascular and cerebrovascular groups (curative ratio: 84% vs. 63.6%, χ2=1.8, P=0.176; mortality: 4% vs. 0%, χ2=0.5, P=0.501). The curative ratios did not show any significant differences between the surgically, conservatively and interventionally treated patients of the cardiovascular group (80% vs. 80% vs. 100%, χ2 = 1.2, P=0.551) (Table 5).
Logistic regression analysis showed significant regressions between bovine trunk and left VA (P=0.000) of the whole setting, and between the dual origins of VAs and cerebral infarct/thrombus (P=0.041), between associated alternative congenital vascular variants and cervical/aortic dissection/atherosclerosis (P=0.008) of the patient cohort. No correlation was found between associated alternative congenital vascular variants and VA dissection/stenosis (P=0.792) or between dual origins of VA and female gender (P=0.788).
Multiple logistic regression demonstrated that side of the aberrant origin of VA (left VA) (P=0.014), arch branch pattern (direct arch origin) (P=0.019), presence of the common trunk (P=0.019), associated acquired vascular disorder (P=0.034) and the patients who warranted management (P=0.000) were significant risk predictors for neurological sequelea; while gender (P=0.512), patient's age (P=0.069), aberrant origin of VA (P=0.075), hypoplasia of VA (P=0.669) and abnormality of VA (P=0.944) were not.
DISCUSSION
Incidence
Direct aortic origin of left VA is the most frequent anatomic variant of VA with a prevalence of 2.4-5.8% in several large autopsy series[44], 2.4-2.5% in patients for cerebral angiography for different reasons[4,45] and 5.25% in patients with suspected extracranial cerebrovascular disease by selective 4-vessel angiography[46]. Aberrant right VA is an extremely rare anomaly[47]. Ding et al.[48] reported a cadaver series of 12 VAs with an aberrant origin, which were all presented on the left, arising from the aortic arch (83.3%) (75% of which originated from the arch between the left common carotid and left subclavian arteries), left common carotid (8.3%) and left external carotid arteries (8.3%). Tsai et al.[49] described, in patients with an aberrant right subclavian artery, the overall prevalence of left VA anomaly was 5.9% (6/102) and that of right VA anomaly was 13.7% (14/102). However, the true incidence of an anomalous origin of right VA from the right common carotid artery remains unknown[50]. With reference to the circumference of the subclavian artery, the location of the origin was cranial in 47%, dorsal in 44%, caudal in 6% and ventral in 3% with an even distribution between the cranial and dorsal quadrants[51].
Embryology
The VAs develop between 33 and 55 days during intrauterine life. The VA is normally formed by the longitudinal anastomoses linking the 7 cervical intersegmental arteries. The intersegmental arteries obliterate soon except for the 7th intersegmental artery, which develops into the subclavian artery involving the origin of VA. In a few cases, the anastomosis between the 6th and 7th intersegmental arteries does not develop on the left side and the 6th intersegmental artery remains, and then the left VA is arising from the aortic arch between the left common carotid and subclavian arteries. Cranial migration of the right VA can result in its branching directly of the aortic arch, and migration relative to the right thyrocervical trunk can lead to some of the other variants[52]. Origin of VAs from the aorta suggests that part of the aortic arch arises from the left 7th intersegmental arteries or there was increased absorption of embryonic tissue of the left subclavian artery between origin of aortic arch and the VA[53]. A faulty degeneration of the primitive dorsal aorta and two intersegmental arteries is considered to be responsible for the development of duplicate VAs[54].
Anatomy
Normally, the VA starts above the first rib plane, accounting for 97.1% (99/102) while in a few cases, its origin was below the first rib plane but in the thorax instead accounting for 2.94% (3/102) [48]. Meila et al.[25] reported that 94.2% of left VA originated from left subclavian artery and entered the foramen transversarium at C6 in nearly all cases; and 6.3% of left VA originated from the aortic arch and entered the foramen transversarium either at C4, C5 or C7 but never at C6. Uchino et al.[23] noted that most left VAs with direct aortic origin proximal to the left subclavian artery entered C4 or C5, and all left VAs with direct aortic origin distal to the left subclavian artery entered C7. All right VAs with proximal right subclavian origin entered C5, C4, or C3; whereas the aberrant right VA entered C7. Moreover, the duplicated segments of left VA fuse at the C5-6 level into a single VA, which then enters the foramen transversarium of C5[3,55,56].
Dodevski et al.[57] reported that the VAs on both sides were equal in diameter in 23.3% patients. The right VA was larger in 30% patients, and the left VA was larger than the right in 46.6% patients. Hypoplasia of VA was found in 6.67% patients. In two patients hypoplasia was on the right side, in one patient on the left side, and in one patient bilateral hypoplasia of the vessel on both sides. Matula et al.[58] stated a hypoplastic artery diameter <3.5 mm was found in 16 (6.96%) cases. Trattnig et al.[45] reported 4.78% of hypoplasia was found on the right side and 2.17% on the left side. Patients with hypoplastic VA may have a high probability of posterior circulation stroke, with atherosclerotic susceptibility and ipsilateral lesions in the VA territory[59].
The significance of the common trunk of the arteries has not been properly indicated in the literature. In 1967, Mueller & Hinck[60] described one patient with bilateral subclavian artery obstruction occurring distal to the origin of VAs. Both VAs supplied to the thyrocervical trunks via extensive collateral vessels, which was termed as "thyrocervical steal". The incidence of a common trunk of the VA and thyrocervical trunk originating from the subclavian artery was 0.58%, and the incidence of a common trunk of the right and left VAs and inferior thyroid artery were 0.64% and 0.13%, respectively[37]. Ding et al.[48] presented a case of cadaver whose left VA originated from the left external carotid artery forming a common trunk with the occipital and posterior auricular arteries.
The frequency of duplication of the VA has been identified in 0.72% of cadavers[19]. Prevertebral duplication may occur when a portion of the primitive dorsal aorta persists along with two intersegmental vessels connected to the true VA[61]. Another explanation is a failure of the fifth or sixth intersegmental artery to regress, which adds a further origin to the VA along with the normal 7th segment[55]. On the right side, both segments usually derive from the right subclavian artery. On the left side, the lateral crus of the duplicated artery commonly starts from the left subclavian artery and the medial one from the aortic arch, between the left common carotid artery and the left subclavian artery. There were also reports of duplicated vessels derived from the thyrocervical trunk[62]. When one of the duplicated origins connects with the contralateral VA and the other ends more distally, opens into the basilar artery. The duplication of the VA might be connected with morphological changes of the VA wall[63]. A duplicated VA is a significant predisposing factor of vertebrobasilar cervical artery dissection due to local histological defects or significant hemodynamics alterations[10,64]. Clinically, patients with diagnosed VA duplication can present a variety of symptoms such as vertigo, dizziness or occipital heaviness. A probable explanation for this is that the lumen of the duplicated vessels can be decreased, predisposing it to easier kinking, resulting in posterior circulation insufficiency[65]. The VA can be easily damaged during severe cervical spine injuries with rapid subluxation, deceleration, fracture through the foramen transversarium, or flexion of the cervical spine, i.e., the VA is easy to suffer from trauma, contusion and crushes as a result of cervical spine injuries[66].
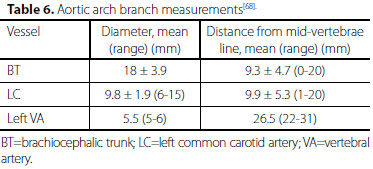
Metrology of the vessels demonstrated that the length of the prevertebral segment of the aberrant left VA was 88.5 mm, and the length of the prevertebral segment of the right VA was 44.3 mm[67]. Aortic arch branch measurements showed that left VA had a smaller diameter but longer distance from the mid-vertebrae line (Table 6).
In only one (11%) cadaver the left VA arose with the left subclavian artery from a common trunk. The trunk originated from the arch behind the left common carotid artery. The diameter of the trunk was 20.0 mm. The distance from its origin to the mid-vertebrae line was 31 mm[68]. The mean distance between the brachiocephalic trunk and left common carotid artery was 0.1-0.5 cm and between the left common carotid artery and left subclavian artery was 0.3-2.0 cm[69]. Some authors tried to depict the anatomy of the dual originated VAs, where the medial limb (3.9 mm in diameter at its origin) originated from its orthodox position, whereas the lateral limb (2.4 mm in diameter at its origin) arose more posteriorly close to the origin of thyrocervical trunk[56].
Diagnosis
Color Doppler sonography is the firstline imaging modality for evaluation of the VAs, although the origin cannot be visualized with this modality in a significant number of patients. Non-visualization of color signals and the absence of spectral tracings will easily establish a diagnosis of occlusion in the extracranial VA segment. In previously reported cases, the diagnosis of a duplicate origin was proven by angiography or, in 1 case, by angiography and magnetic resonance imaging[70]. An abrupt change in the diameter of the VA raised the possibility of a dissection[71]. Diagnostic problems may ensue when a filling defect is created at the junction between the anomalous origin and the normal origin of the VAs from non-opacified blood. An abrupt change in the size of VA at its juncture with a dual origin would suggest hypoplasia or even pathological narrowing of the vessel. With the increasing utilization of digital vascular imaging by way of the venous route, and with a smaller field of view, one may consider the VA to be hypoplastic or even occluded unless the possibility of a bifid origin is considered[31].
Clinical Significance
The patients with left VA variants are usually asymptomatic, or with symptoms resulting from other than the aberrant VA. Rare cases have presented with dizziness, but this does not seem to be associated with the anomalous origin[5]. The patients may be asymptomatic unless the VA is involved by atherosclerotic lesions[72]. The left VA origin anomaly with its C4 entrance and contralateral hypoplasia could cause ataxia during head rotation[5]. Symptoms of patients with duplicated VA are probably not related to these anomalies of VAs[62]. The significance of recognizing left lateral medullary infarction associated with mild intracranial VA stenosis[22]. The anomalous VA origin may be an independent risk factor for arterial dissection; the longer extra-cranial course may lead to increased vulnerability of the vessel wall to shear stress resulting in intimal tear and dissection[11]. Headaches and neurological symptoms in a patient with an anomalous VA origin should initiate a thorough investigation for arterial dissection[11].
Komiyama et al.[4] in their study detected arterial dissection in 17 patients with an incidence of 1.9%. They analyzed that the left VA of aortic origin showed a remarkably higher incidence of arterial dissection than left VA of a left subclavian and right VA of a right subclavian artery origin. The reasons for the high incidence of arterial dissection associated with VA of aortic origin remain to be elucidated. An anomalous left VA arising directly from the aortic arch typically enters the C4 or C5 foramen transversarium, resulting in a longer course of VA in the neck, thus predisposing to VA dissection. The present study revealed an incidence of VA dissection of 0.9% (9/955).
VA morphological variants determine regional hemodynamic solution[66]. The VA blood flow volume accounts for 31% of the total brain flow volume at the age of 4 years, and declines significantly thereafter until the age of 18 years. After this age the blood flow volume percentage of VAs stabilizes at 24%[66].
There was no association between a bovine trunk and direct origin of left VA[73]. Also, there was no evidence of association between a dual left VA origin and distal vertebral thromboembolism, or between other vascular anomaly and premature atherosclerosis or intracranial dissection[22]. The incidence of duplicated VA among the patients for computed tomography angiography was 0.74%, all occurring in female patients[25]. Contrary to the above-mentioned, the present study revealed close relations between bovine trunk and left VA, dual origins of VAs and cerebral infarct/thrombus formation, and between associated alternative congenital vascular variants and cervical/aortic dissection/atherosclerosis, but no female gender predilection for dual origins of VA. Moreover, aberrant origin of left VA and aberrant origin of right subclavian artery were frequent in Down syndrome patients. In return, aberrant origin of left VA and right subclavian artery might be helpful in the diagnosis of Down syndrome[74].
The usual procedures of endarterectomy of the proximal left VA or even transplantation to the left common carotid artery may not be necessary if the true anatomic configuration is identified[31]. The isolated left VA was reconstructed with a saphenous vein graft interposed between the native isolated left VA and the side of the graft branch anastomosed to the left subclavian artery[6].
The patient management using an antiplatelet regimen is an alternative for these patients[75]. As an aortic arch surgery might be complicated by ischemic issues, which can be caused by unrecognized variation of its vascular anatomy[68], accurate assessment of anatomic variations of VAs is recommended before aortic arch surgery or endovascular interventions[5]. Care has to be taken when planning to cover the origin of left subclavian artery[76]. Detailed knowledge of an anomalous origin of supraaortic arteries is also of importance for patients who have to undergo 4-vessel angiography[77]. A duplicated VA influences surgical procedures performed on the head and neck region. Visualization of only one trunk of a double VA during catheterization can lead to misdiagnosing the VA as hypoplastic[78]. During surgical incision of the muscles of the transverse spinal processes (deep cervical region) one may damage an abnormally long prevertebral VA segment (V1)[66].
CONCLUSION
The patients with neurological symptoms and those for neck and chest operations/interventions should be carefully screened for the possibility of an aberrant origin of VA. The results from the cadaver metrology study are very helpful in the design of the aortic stent. The arch branch pattern has to be taken into consideration before any maneuver in the local region so as to avoid unexpected events in relation to aberrant VAs.
REFERENCES
1. Rozin L, Rozin R, Koehler SA, Shakir A, Ladham S, Barmada M, et al. Death during transforaminal epidural steroid nerve root block (C7) due to perforation of the left vertebral artery. Am J Forensic Med Pathol. 2003;24(4):351-5. [MedLine]
2. Polguj M, Podgórski M, Jedrzejewski K, Topol M, Majos A. Fenestration and duplication of the vertebral artery: the anatomical and clinical points of view. Clin Anat. 2013;26(8):933-43. [MedLine]
3. Almeida BL, Kambara AM, Rossi FH, Moreira SM, Oliveira ES, Linhares Filho FA, et al. Left subclavian artery stenting: an option for the treatment of the coronary-subclavian steal syndrome. Rev Bras Cir Cardiovasc. 2014;29(2):236-40. [MedLine]
4. Komiyama M, Morikawa T, Nakajima H, Nishikawa M, Yasui T. High incidence of arterial dissection associated with left vertebral artery of aortic origin. Neurol Med Chir (Tokyo). 2001;41(1):8-12.
5. Gabrielli R, Rosati MS. Ataxia and vertigo due to anomalous origin of the left vertebral artery. J Vasc Surg. 2013;58(3):803. [MedLine]
6. Yamashiro S, Kuniyoshi Y, Arakaki K, Inafuku H, Morishima Y, Kise Y. Total arch replacement with associated anomaly of the left vertebral artery. Ann Thorac Cardiovasc Surg. 2010;16(3):216-9. [MedLine]
7. Lacout A, Khalil A, Figl A, Liloku R, Marcy PY. Vertebral arteria lusoria: a life-threatening condition for oesophageal surgery. Surg Radiol Anat. 2012;34(4):381-3. [MedLine]
8. Komiyama M, Nakajima H, Yamanaka K, Iwai Y. Dual origin of the vertebral artery: case report. Neurol Med Chir (Tokyo). 1999;39(13):932-7. [MedLine]
9. Chen CJ, Wang LJ, Wong YC. Abnormal origin of the vertebral artery from the common carotid artery. AJNR Am J Neuroradiol. 1998;19(8):1414-6. [MedLine]
10. Dare AO, Chaloupka JC, Putman CM, Mayer PL, Schneck MJ, Fayad PB. Vertebrobasilar dissection in a duplicated cervical vertebral artery: a possible pathoetiologic association? A case report. Vasc Endovascular Surg. 1997;31(1):103-9.
11. Dudich K, Bhadelia R, Srinivasan J. Anomalous vertebral artery origin may be an independent risk factor for arterial dissection. Eur J Neurol. 2005;12(7):571-2. [MedLine]
12. Goddard AJ, Annesley-Williams D, Guthrie JA, Weston M. Duplication of the vertebral artery: report of two cases and review of the literature. Neuroradiology. 2001;43(6):477-80. [MedLine]
13. Kimura K, Yonemitsu M, Hashimoto Y, Uchino M. Spontaneous dissection associated with proximal vertebral artery anomaly. Intern Med. 1997;36(11):834-6. [MedLine]
14. Koenigsberg RA, Pereira L, Nair B, McCormick D, Schwartzman R. Unusual vertebral artery origins: examples and related pathology. Catheter Cardiovasc Interv. 2003;59(2):244-50. [MedLine]
15. Layton KF, Miller GM, Kalina P. Aberrant origin of the right vertebral artery from the right common carotid artery: depiction of a rare vascular anomaly on magnetic resonance angiography. J Vasc Interv Radiol. 2006;17(6):1065-7. [MedLine]
16. Liu Y, Hua Y, Liu B, Jia L, Jiao L. Anomalous origin of bilateral vertebral arteries from the ICA: review of the literature and a case report. Ann Vasc Surg. 2014;28(5):1319.e13-6. [MedLine]
17. Mordasisni P, Schmidt F, Schroth G, Remonda L. Asymmetrical bilateral duplication of the extracranial vertebral arteries: Report of a unique case. Eur J Radiol Extra. 2008;67:e91-4.
18. Patil PV, Patil AM, Apte AV, Attarde VY. Anomalous origin of left vertebral artery from carotid bulb seen as “trifurcation” of left common carotid artery with acute infarct in ipsilateral thalamus: a case report. J Neuroimaging. 2015;25(4):662-4. [MedLine]
19. Polguj M, Jedrzejewski K, Topol M, Wieczorek-Pastusiak J, Majos A. Duplication of the left vertebral artery in a patient with dissection of the right internal carotid artery and Ehlers-Danlos syndrome: case report and review of the literature. Anat Sci Int. 2013;88(2):109-14. [MedLine]
20. Taha MM, Nakahara I, Higashi T, Iwamuro Y, Watanabe Y, Taki W. Percutaneous angioplasty and stenting of subclavian arteries before surgical coronary revascularization in a patient with an aberrant right subclavian artery. J Neuroradiol. 2007;34(4):267-71. [MedLine]
21. Takasato Y, Hayashi H, Kobayashi T, Hashimoto Y. Duplicated origin of right vertebral artery with rudimentary and accessory left vertebral arteries. Neuroradiology. 1992;34(4):287-9. [MedLine]
22. Tobin WO, Killeen R, Kinsella JA, McCabe DJ. Dual origin of the left vertebral artery: extracranial MRA and CTA findings. J Neurol Sci. 2010;298(1-2):150-2. [MedLine]
23. Uchino A, Saito N, Takahashi M, Okada Y, Kozawa E, Nishi N, et al. Variations in the origin of the vertebral artery and its level of entry into the transverse foramen diagnosed by CT angiography. Neuroradiology. 2013;55(5):585-94. [MedLine]
24. Yanik B, Conkbayir I, Keyik B, Hekimoglu B. A rare anomalous origin of right vertebral artery: findings on Doppler sonography. J Clin Ultrasound. 2004;32(4):211-4. [MedLine]
25. Meila D, Tysiac M, Petersen M, Theisen O, Wetter A, Mangold A, et al. Origin and course of the extracranial vertebral artery: CTA findings and embryologic considerations. Clin Neuroradiol. 2012;22(4):327-33. [MedLine]
26. Thomas AJ, Germanwala AV, Vora N, Prevedello DM, Jovin T, Kassam A, et al. Dual origin extracranial vertebral artery: case report and embryology. J Neuroimaging. 2008;18(2):173-6. [MedLine]
27. Tuncel SA, Çagli B, Sengül E, Ünlü E. Anomalous origin of both vertebral arteries combined with aberrant right subclavian artery and truncus bicaroticus. Nobel Med. 2012;8(3):124-6.
28. Tummala RP, Ecker RD, Levy EI. Variant of subclavian steal in the setting of ipsilateral common carotid artery occlusion: case report. J Neuroimaging. 2009;19(3):271-3. [MedLine]
29. Albayram S, Gailloud P, Wasserman BA. Bilateral arch origin of the vertebral arteries. AJNR Am J Neuroradiol. 2002;23(3):455-8. [MedLine]
30. Cheng M, Xiaodong X, Wang C, You C, Mao B, He M, et al. Two anatomic variations of the vertebral artery in four patients. Ann Vasc Surg. 2009;23(5):689.e1-5. [MedLine]
31. Eisenberg RA, Vines FS, Taylor SB. Bifid origin of the left vertebral artery. Radiology. 1986;159(2):429-30. [MedLine]
32. Flynn RE. External carotid origin of the dominant vertebral artery: case report. J Neurosurg. 1968;29(3):300-1. [MedLine]
33. Goldstein S. Aberrant right subclavian artery and right vertebral artery with ACA aneurysm. MyPACS.net: Radiology Teaching Files > Case 16846. Accessed on March 11, 2015. Available at: http://www.mypacs.net/cases/aberrant-right-subclavian-artery-and-right-vertebral-artery-with-aca-aneurysm-16846.html
34. Ishihara H, San Millan Ruiz D, Abdo G, Asakura F, Yilmaz H, Lovblad KO, et al. Combination of rare right arterial variation with anomalous origins of the vertebral artery, aberrant subclavian artery and persistent trigeminal artery. A case report. Interv Neuroradiol. 2011;17(3):339-42. [MedLine]
35. Nalamada K, Chitravanshi N, Duffis EJ, Prestigiacomo CJ, Gandhi CD. Anomalous origin of the right vertebral artery from the right common carotid artery associated with an aberrant right subclavian artery. J Neurointerv Surg. 2013;5(5):e34. [MedLine]
36. Nasir S, Hussain M, Khan SA, Mansoor MA, Sharif S. Anomalous origin of right vertebral artery from right external carotid artery. J Coll Physicians Surg Pak. 2010;20(3):208-10. [MedLine]
37. Sartor K, Freckmann N, Böker DK. Related anomalies of origin of left vertebral and left inferior thyroid arteries: report of three cases. Neuroradiology. 1980;19(1):27-30. [MedLine]
38. Satti SR, Cerniglia CA, Koenigsberg RA. Cervical vertebral artery variations: an anatomic study. AJNR Am J Neuroradiol. 2007;28(5):976-80. [MedLine]
39. Sugita S, Abe T, Okura A, Wantanabe M, Shigemori M. MRI demonstration of double origin of the left vertebral artery: case note. Neuroradiology. 1995;37(4):295-6. [MedLine]
40. Kumar S, Moses E, Mishra NK, Nanda A. Right vertebral artery originating from the aorta distal to left subclavian artery and ending in posterior inferior cerebellar artery in a patient with Moya-Moya disease. Clin Neuroradiol. 2008;18(3):187-90.
41. Lotfi M, Nabavizadeh SA, Foroughi AA. Aortic arch vessel anomalies associated with persistent trigeminal artery. Clin Imaging. 2012;36(3):218-20. [MedLine]
42. Ligege P, Scholtz L. Rare variation in the origin of the right vertebral artery. S A J Radiol. 2004;8(1):34-5.
43. Kim YD, Yeo HT, Cho YD. Anomalous variations of the origin and course of vertebral arteries in patients with retroesophageal right subclavian artery. J Korean Neurosurg Soc. 2009;45(5):297-9. [MedLine]
44. Al-Okaili R, Schwartz ED. Bilateral aortic origins of the vertebral arteries with right vertebral artery arising distal to left subclavian artery: case report. Surg Neurol. 2007;67(2):174-6.
45. Trattnig S, Matula C, Karnel F, Daha K, Tschabitscher M, Schwaighofer B. Difficulties in examination of the origin of the vertebral artery by duplex and colour-coded Doppler sonography: anatomical considerations. Neuroradiology. 1993;35(4):296-9. [MedLine]
46. Palmer J. Anomalous origin of the left vertebral artery and its significance in selective femoro-cerebral catheterisation. Australas Radiol. 1976;20(3):225-8. [MedLine]
47. Canyigit M, Akgoz A, Koksal A, Yucesoy C. Aberrant right vertebral artery: a rare aortic arch anomaly. Br J Radiol. 2009;82(981):789-91. [MedLine]
48. Ding JM,Yu QB,Zhang H. Anatomy and its origins variation researches of the prevertebral part for vertebral artery. J North Sichuan Med Coll. 2004;19(4):10-1.
49. Tsai IC, Tzeng WS, Lee T, Jan SL, Fu YC, Chen MC, et al. Vertebral and carotid artery anomalies in patients with aberrant right subclavian arteries. Pediatr Radiol. 2007;37(10):1007-12. [MedLine]
50. Park JK, Kim SH, Kim BS, Choi G. Two cases of aberrant right subclavian artery and right vertebral artery that originated from the right common carotid artery. Korean J Radiol. 2008;9(Suppl):S39-42. [MedLine]
51. Dodevski A, Tosovska-Lazarova D. Anatomical features and clinical importance of the vertebral artery. Macedonian J Med Sci. 2012;5(3):328-35.
52. Wasserman BA, Mikulis DJ, Manzione JV. Origin of the right vertebral artery from the left side of the aortic arch proximal to the origin of the left subclavian artery. AJNR Am J Neuroradiol. 1992;13(1):355-8. [MedLine]
53. Manyama M, Rambau P, Gilyoma J, Mahalu W. A variant branching pattern of the aortic arch: a case report. J Cardiothorac Surg. 2011;6:29. [MedLine]
54. Gaskill SJ, Heinz ER, Kandt R, Oakes WJ. Bilateral congenital anomalies of the extracranial vertebral artery: management with balloon therapy. Pediatr Neurosurg. 1996;25(3):147-50. [MedLine]
55. Kendi AT, Brace JR. Vertebral artery duplication and aneurysms: 64-slice multidetector CT findings. Br J Radiol. 2009;82(983):e216-8. [MedLine]
56. Rameshbabu C, Gupta OP, Gupta KK, Qasim M. Bilateral asymmetrical duplicated origin of vertebral arteries: multidetector row CT angiographic study. Indian J Radiol Imaging. 2014;24(1):61-5. [MedLine]
57. Dodevski A, Lazareska M, Tosovska-Lazarova D, Zhivadinovik J, Aliji V. Morphological characteristics of the first part of the vertebral artery. Prilozi. 2011;32(1):173-88. [MedLine]
58. Matula C, Tratting S, Tscabitscer M, Day JD, Koos WT. The course of the prevertebral segment of the vertebral artery: anatomy and clinical significance. Surg Neurol. 1997;48(2):125-31. [MedLine]
59. Park JH, Kim JM, Roh JK. Hypoplastic vertebral artery: frequency and associations with ischaemic stroke territory. J Neurol Neurosurg Psychiatry. 2007;78(9):954-8. [MedLine]
60. Mueller RL, Hinck VC. Thyrocervical steal. Am J Roentgenol Radium Ther Nucl Med. 1967;101(1):128-9. [MedLine]
61. Vasovic LP. Reevaluation of the morphological parameters according to 11 different duplications of the fetal vertebral artery at prevertebral (V1) and intracranial (V4) parts. Cells Tissues Organs. 2004;176(4):195-204. [MedLine]
62. Kiss J. Bifid origin of the right vertebral artery: a case report. Radiology. 1968;91(5):931. [MedLine]
63. Harnier S, Harzheim A, Limmroth V, Horz R, Kuhn J. Duplication of the common carotid artery and the ipsilateral vertebral artery with a fenestration of the contralateral common carotid artery. Neurol India. 2008;56(4):491-3. [MedLine]
64. Melki E, Nasser G, Vandendries C, Adams D, Ducreux D, Denier C. Congenital vertebral duplication: a predisposing risk factor for dissection. J Neurol Sci. 2012;314(1-2):161-2. [MedLine]
65. Ionete C, Omojola MF. MR angiographic demonstration of bilateral duplication of the extracranial vertebral artery: unusual course and review of the literature. AJNR Am J Neuroradiol. 2006;27(6):1304-6. [MedLine]
66. Giuffrè R, Sherkat S. Maldevelopmental pathology of the vertebral artery in infancy and childhood. Childs Nerv Syst. 2000;16(10-11):627-32. [MedLine]
67. Imre N, Yalcin B, Ozan H. Unusual origin of the left vertebral artery. Int J Anat Variat. 2010;3:80-2.
68. Alsaif HA, Ramadan WS. An anatomical study of the aortic arch variations. JKAU: Med Sci. 2010;17(2):37-54.
69. Amer AH. Variability of aortic arch branching. Accessed on March 11, 2015. Available at: http://bsclupan.asm.md:8080/xmlui/bitstream/handle/123456789/561/11.pdf?sequence=1.
70. Mahmutyazicioglu K, Saraç K, Bölük A, Kutlu R. Duplicate origin of left vertebral artery with thrombosis at the origin: color Doppler sonography and CT angiography findings. J Clin Ultrasound. 1998;26(6):323-5. [MedLine]
71. Nogueira TE, Chambers AA, Brueggemeyer MT, Miller TJ. Dual origin of the vertebral artery mimicking dissection. AJNR Am J Neuroradiol. 1997;18(2):382-4. [MedLine]
72. Phan T, Huston J 3rd, Bernstein MA, Riederer SJ, Brown RD Jr. Contrast-enhanced magnetic resonance angiography of the cervical vessels: experience with 422 patients. Stroke. 2001;32(10):2282-6. [MedLine]
73. Berko NS, Jain VR, Godelman A, Stein EG, Ghosh S, Haramati LB. Variants and anomalies of thoracic vasculature on computed tomographic angiography in adults. J Comput Assist Tomogr. 2009;33(4):523-8. [MedLine]
74. Rathore MH, Sreenivasan VV. Vertebral and right subclavian artery abnormalities in the Down syndrome. Am J Cardiol. 1989;63(20):1528-9. [MedLine]
75. Chahwan S, Miller MT, Kim KA, Mantell M, Kirksey L. Aberrant right subclavian artery associated with a common origin of carotid arteries. Ann Vasc Surg. 2006;20(6):809-12. [MedLine]
76. Dakota I. Acute limb ischemia following TEVAR in patient with anomalous origin of left vertebral artery. Accessed on March 11, 2015. Available at: http://summitmd.com/pdf/pdf/2000_Dakota.pdf
77. Lemke AJ, Benndorf G, Liebig T, Felix R. Anomalous origin of the right vertebral artery: review of the literature and case report of right vertebral artery origin distal to the left subclavian artery. AJNR Am J Neuroradiol. 1999;20(7):1318-21. [MedLine]
78. Kim DW. Concomitant dual origin and fenestration of the left vertebral artery resembling dissection. J Korean Neurosurg Soc. 2009;46(5):498-500. [MedLine]
No financial support.
Author's roles & responsibilities
SMY Study conception and design; analysis and/or interpretation of data; manuscript writing, final approval of the manuscript
Article receive on Thursday, July 2, 2015
 All scientific articles published at www.rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at www.rbccv.org.br are licensed under a Creative Commons license