Ovandir Bazan0; Jayme Pinto Ortiz0
DOI: 10.1590/S0102-76382011000200009
ABSTRACT
BACKGROUND: Since most complications related to the operation of
prosthetic heart valves is due to disturbances of flow, its hydrodynamic
characterization is a useful aid in the design of new prostheses. Simulations
of pulsatile flow in cardiac prostheses began nearly 40 years ago, through the
development of different mock human circulatory systems, improving the clinical
results interpretation. A new design of a pulse duplicator system was developed
at Polytechnic School of USP to study prosthetic heart valves.
OBJECTIVE: To present the conception of a new mock circulatory system
for hydrodynamic simulations of cardiac prosthetic valves and the assembly plan
of an experiment whose focus is the test of mitral prosthesis.
METHODS: Its conception is based on the state-of-art's review of these
studies and the experience got with the previous mock circulatory systems,
particularly the one used in the Instituto Dante Pazzanese de Cardiologia,
São Paulo, SP, Brazil.
RESULTS: In this design, an electric servomotor controlled by computer
emits, through a hydraulic piston, a pulse to the left ventricular chamber
model, where the heart valves are accomodated. To characterize, in the future,
the dynamic operation of mitral prosthetic valves, an experimental setup was
mounted to provide measurements of volumetric flow, instantaneous pressure and
velocity fields on these valves. Optical access is conveniently provided on the
design, making possible the use, in the future, of a LDA system.
CONCLUSIONS: In order to improve the analysis of hydrodynamic shear
stress and prediction of haemolysis, the experimental results may be used to
regulate a numerical model using 'Computational Fluid Dynamics' (CFD).
RESUMO
INTRODUÇÃO: Uma vez que a maioria das complicações
relacionadas ao funcionamento das próteses de válvulas
cardíacas é decorrente de distúrbios de escoamento, a sua
caracterização hidrodinâmica é um auxílio
útil no projeto de novas próteses. Simulações do
escoamento pulsátil em próteses cardíacas começaram
há cerca de 40 anos, por meio do desenvolvimento de diferentes bancadas
do sistema circulatório humano, melhorando a interpretação
dos resultados clínicos. Um novo projeto de um sistema duplicador de
pulsos foi desenvolvido na Escola Politécnica da USP para estudar
próteses de válvulas cardíacas.
OBJETIVO: Apresentar a concepção da nova bancada
experimental de fluxo pulsátil para ensaios hidrodinâmicos de
próteses de válvulas cardíacas e o plano de montagem de um
experimento cujo foco é o ensaio de próteses mitrais.
MÉTODOS: Sua concepção é baseada na
revisão do estado da arte desses estudos e na experiência obtida
nas bancadas do sistema circulatório, particularmente aquela usada no
Instituto Dante Pazzanese de Cardiologia, em São Paulo, Brasil.
RESULTADOS: Neste projeto, um servomotor elétrico controlado por
computador emite, por meio de um pistão hidráulico, um pulso para
o modelo da câmara do ventrículo esquerdo, onde as válvulas
cardíacas são acomodadas. Para caracterizar, no futuro, a
operação dinâmica das próteses de válvulas
mitrais, foi montado um experimento para proporcionar medições de
vazão volumétrica, pressão instantânea e campos de
velocidade nessas válvulas. Acessos ópticos estão
convenientemente previstos no projeto, tornando possível o uso, no
futuro, de um sistema LDA.
CONCLUSÕES: A fim de melhorar a análise das tensões
hidrodinâmicas e a previsão de hemólise, os resultados
experimentais podem ser utilizados para ajustar um modelo numérico
usando 'Computational Fluid Dynamics' (CFD).
INTRODUCTION
Some of the diseases of the cardiovascular system are originated by the malfunction of the heart valves, which seriously interfere with the ability to pump blood.
Since the first successful implant of a prosthetic heart valve, about 50 different biological and mechanical models were designed [1].
All prosthetic heart valves cause narrowing of the valve orifice, which arises from the thickness of its ring and, moreover, a certain transvalvular pressure gradient occurs in all valves and has an inverse relation to blood flow through the valve orifice, in addition to depending on size, type and model of the valve. Mechanical prostheses have on their biological valves the advantage of long life, but patients who use them are still subject to thrombosis and thromboembolism, even if they are properly treated with anticoagulants and antiplatelet agents [2-4].
The biological valves require the use of anticoagulants, but their durability is limited as a result of degeneration. Porcine bioprosthesis can be expected to last for 15 years in the aortic position and up to 10 years in the mitral position, it is also recommended for elderly patients and those who cannot receive anticoagulants [5]. There are advantages and disadvantages in the use of biological or mechanical prostheses and both must be available to the patient.
Since most of the complications related to the operation of cardiac prosthetic valves is due to flow disturbances [6,7], their hydrodynamic characterization is a very important aid in the design of new prostheses [8,9]. Velocity profiles and stresses are different for each type of valve, which can occur in regions of stagnation and separation of the fluid, allowing the formation of thrombosis, tissue overgrowth and / or calcifications, and hemolysis of blood due to shear stress [8,10].
Studies with simulations of pulsatile flow in heart prostheses are very important because they provide results of much scientific and clinical interest. These studies are based on the development of several bench pulse duplicating projects [11-13], associated with 2D/3D measurement techniques to characterize the hydrodynamic functioning of the valves [8,10,14-18]. For example, you can attach to these benches a pulse duplicating system for Particle Image Velocimetry (or PIV), often used to map the velocity field in a non-invasive manner [16]. Another technique is laser velocimetry (Laser Doppler Anemometer, or LDA), able to map accurately and timely disposal in a non-invasive manner and enable the framework of the scales of turbulence flow [1,19], helping in the characterization of possible hemolysis.
In most current bench designs, in addition to replicate the physiological human pulse, it is aimed somehow to have the geometry and the confinement of the valves, as it occurs in humans. Thus, it is also possible to study the flow direction regarding the vortices (or whirls) generated by the flow and analyze the influence of hydrodynamic instabilities that are transferred from one valve to another [11-13].
To aid the analysis of hydrodynamic stresses and prediction of possible rates of hemolysis, the experimental results are often used to set a numerical model for Computational Fluid Dynamics (CFD), capable of performing, for example, simulations involving fluid characteristics of the flow trajectories, the velocity field, the gradients of pressure, tension and formation of vortices [9,12].
Assessment of costs in the country
In Brazil, the economics profoundly shape the choice of valve type: 80% of the prostheses implanted are biological. This is because the SUS (Unified Health System) paid by each biological valve approximately R$ 950.00, while the double-leaflet mechanical prostheses cost the State around R$ 3,690.00 (current prices in February 4, 2010) [1]. In addition, patients with mechanical valves require anticoagulant medication continuously, which costs the patient about R$250.00 to R$ 450.00 a year, not counting the cost of medical follow-up.
Brazil is a major producer of heart valves in the world and by 2002 this market generated more than US$10 million a year, having as main manufacturers Braile Biomédica S/A, Brazil, St. Jude Medical and Labcor. About 8 thousand to 10 thousand patients annually receive cardiac valve prostheses both biological and mechanical in the country [20].
Qualification of the main problem to be addressed
Studies with simulations of pulsatile flow in cardiac prostheses have been used for nearly 40 years, through the development of several pulse duplicating bench projects , such as " Yoganathan-FDA system", "Aachen pulse duplicator", "Sheffield pulse duplicator" and "Vivitro pulse duplicator" [14].
During 2009, the weekly monitoring using the bench pulsatile flow simulation by the Bioengineering Dept. of the Dante Pazzanese Institute of Cardiology (IDPC) in São Paulo, Brazil, helped the introduction and manipulation of this type of equipment [21,22]. This bench is capable of creating an in vitro environment to evaluate the performance of the circulatory assistance device (mimicking the physiological system) by the simulation of the complacency of arteries and peripheral resistance (elastance of the heart).
However, for the investigations of the Biomedical Engineering group (PME / EPUSP) [23.24] were directed to specific scientific and clinical interest in heart valves flow, it had to meet all requirements of ISO 5840:2005 (Cardiovascular Implants - Cardiac Valve Prostheses) for pulsatile flow regime [25]. Basically, it would implicate providing the IDPC bench of optical access for laser anemometry, adapting it for an interchange of valves and make it able to meet a higher pulsatile flow. Especially for the latter aspect, it was deemed an appropriate final design of a bench in the EPUSP. Therefore, new alternatives for countertops that have been used domestically and abroad were sought [11-14] and such a system was designed for the modeling study of flow in heart prosthetic valves, able to study the performance of prostheses in aortic and mitral positions. Currently, the project is complete, this bench is under construction.
OBJECTIVE
The objective of this paper is to present the design of the experimental bench of pulsatile flow for hydrodynamic testing of mitral and aortic prosthetic valves whose design phase has been completed. It is also presented the assembly plan of an experiment whose focus is the test of mitral prostheses that may in fact be carried out when the bench is in operation.
The high frequency of mitral valve replacement, and the vast literature reporting hydrodynamic studies in that position, reinforced our choice to restrict the focus of the work and initially simulate mitral prosthetic heart valves [26].
The construction phase of the bench and the actual trial in vitro of the prostheses will be discussed in subsequent papers. Still subsequently, the experimental results obtained in the simulations will be used in setting a computational model [8] and compared with the physiological hemodynamic and human pathophysiological literature, so that they are determined, for each type of mitral valve, the problems that occur in them, particularly with regard to possible hemolysis of the blood [1,9,17,19,27]. Nothing prevents other studies on the same bench can stick to the aortic position in the future.
METHODS
In order to achieve the real goal of this and experimental bench design work and the design of an initial experiment to test mitral valves, the methodology used involves the overall design of a human physiologic pulse duplicating bench (simulating the left side of the heart) and the determination of equipment and instrumentation to be incorporated into the bench in the final phase of its construction in order to simulate experimental hydrodynamics.
Testing bench for hydrodynamic simulation
The final design of the testing bench (SME / EPUSP) is based on experience in monitoring the activities of the Dante Pazzanese Institute of Cardiology with artificial hearts in master's and doctoral works previously developed by Silva et al. [23], Leal [24], Bessa & Ortiz [28] and Legendre [29], adapting them to pulsatile flow through prosthetic heart valves and in the literature review on the subject. In particular, it meets the requirements of international standard ISO 5840:2005 for pulsed flow regime [25] and includes the views of the benches using the deformable model of the left ventricle (silicone) and the disposition of the prosthetic valves in geometries similar those occurring in vivo [11-13]. The place for accommodation of the valves in the bench is scaled to meet all standard sizes of aortic and mitral prostheses.
Mounting an experiment to test mitral valve
In view of the hydrodynamic experimentation in mitral prosthetic heart valves, as soon as the bench is completely built, it must be incorporated equipment for measuring pressure, flow and temperature as well as a data acquisition system, so that it can be calibrated with a physiological pulse rate. In the subsequent stage, measurements shall be performed to characterize the hydrodynamic velocity profiles and stresses in the mitral valve, through a system of laser anemometry (LDA). Hence, the instrumentation includes four pressure transducers, a temperature controller, an ultrasonic flow meter (not invasive, so as not to interfere with the flow), a data acquisition system and a system of laser anemometry. It also requires a computer in order to perform the servo drive control of the experimental bench and data acquisition.
The test fluid to be used on the bench shall be able to simulate both the density and viscosity characteristics of blood. The importance of checking the index of refraction of the test fluid is that this coefficient is critical in directing the laser beams.
The sampling requirements and the simulation of the valves on the bench must meet the requirements of ISO 5840:2005 for pulsatile flow regime.
In order to give subsidies to the interpretation of results and help in optimization of these prostheses, the information obtained in the experimental simulations will be used to adjust a computer model [28].
RESULTS
The new bench pulse duplicator (PME/EPUSP) was designed to meet the clinical and scientific interest focused on the characterization of flow in prosthetic heart valves. According to ISO 5840:2005, it has settings for ventricular ejection volume, heart rate and cardiac output.
It is designed so that the possible arrangement of components facilitates maintenance. Currently, the focus of study is the mitral position. Figure 1 shows, schematically, the main components of this bench. Figure 2 shows, in Unigraphics/NX5 (PACE / SME / EPUSP) environment, the three-dimensional modeling of the main components assembled as a result of the dynamic functional and structural design to meet the issue of physiological pulses.


The operating principle is based on this side of the left ventricular function of the heart. However, it was designed to use two fluids: the working fluid (responsible for transmitting the pulse to the model of the ventricle) and the test fluid (which simulates the properties of the blood and travels in a hydraulic circuit bench, passing through the prosthesis). The working fluid and the test fluid are separated by a flexible silicone membrane, which delimits the bottom of the model of the left ventricle. The other part of the ventricle is stiff, its upper limits, where the aortic and mitral prostheses will be lodged. This rigid top, called "prosthesis fixation platform " ("PF" in Figure 1) has optical access to allow penetration of the laser beams of the LDA system. Thus, the complete model of the ventricle, consisting of the flexible membrane and fixing the platform of the prostheses, can be said "deformable left ventricle" ("DLV" in Figure 1). Thus, the ventricular chamber is composed by the inner walls of the flexible membrane and fixation platform of the prostheses.
For the operation of this bench, a computer-controlled servo drive is coupled to a linear table which, in turn, transmits the reciprocating motion of a piston within a cylinder. When the movement of the piston increases the pressure inside the global tank, a pulse is transmitted through the working fluid to the flexible membrane of the deformable left ventricle. This causes the membrane to be retracted, simulating ventricular systole. At the same time within the ventricular chamber, the retraction of the membrane transmits an increase of pressure to the portion of the test fluid which is confined there since the valves are closed.
With the addition of pressure in the ventricular chamber, the mitral valve remains closed; however, when the system pressure is overcome, the aortic valve opens and allows the test fluid stored in the ventricular chamber to drain to other components of the bench. This lasts until the end of ventricular ejection. As the cylinder piston returns and starts the ventricular diastole, the pressure in the model of the ventricular chamber will decrease until the instant it becomes lower than the system pressure, the, the aortic valve closes and the mitral valve opens, causing the test fluid stored in the model of the atrium to drain into the ventricular chamber.
In the design of the trial bench, the geometries, dimensions and disposition of the prostheses in their fixation platform were based on the anatomy of the left heart. The lodging of the valves is also scaled to meet the mounting of all standard nominal diameters for the aortic and mitral prostheses according to ISO 5840:2005. The pulse emitted by the piston can be adjusted to simulate specific elastance of the heart. The bench is also equipped with two adjustable modules of complacency, ventricular compliance and peripheral resistance. For example, adjustments in ventricular compliance will allow adjusting the interference of flexibility of the membrane (of the deformable left ventricle) in the system in order to contribute to the physiological adjustment of the physiological pulse.
The platform for fixation of the prosthesis is designed so that optical access was disposed in order to permit the future use of the LDA in both the aortic and mitral position.
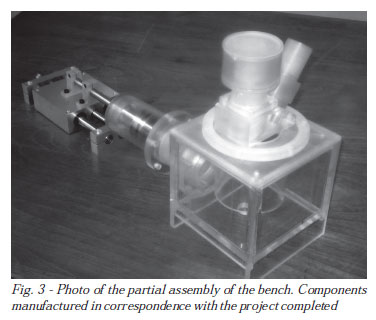
Currently, completed the design phase, the testing bench is being built. Figure 3 shows a partial picture of the bench assembly that includes several components already manufactured.
DISCUSSION
This discussion addresses some design details in setting up an experiment that involves experimental and computational simulation of pulsatile flow through mitral cardiac prostheses. The actual implementation of this experiment can be started as soon as the designed pulse duplicating bench is fully constructed and instrumented. Then, after setting a physiological pulse on the bench, it will be replicated through simulations of prosthetic mitral valves in the fields of pressure, velocity and shear stresses, which will be recorded in data acquisition and laser velocimetry. In the subsequent step, these experimental data will be used to adjust a computer model that allows interpreting all results. This experimental design is outlined in the following steps.
Preliminary Preparations
Once the bench is fully built, it will be allocated at the Laboratory of Biomedical and Environmental Engineering (LAB) of the Department of Mechanical Engineering - EPUSP. In it will be allocated equipment for measuring pressure, flow, speed and temperature as well as a system of data acquisition and servo drive control via computer.
As is illustrated in Figure 1, the working fluid will occupy the entire volume that includes the cylinder and the inner walls of the global reservoir. The test fluid will occupy the entire volume contained by the inner walls of the chamber and the ventricular system. Mechanisms are arranged on the bench for the leakage and measurement of air where the work and test fluids are confined.
Based on the experience of previous works and literature, for this study it shall be used a solution of 1/3 glycerin, 1/3 water and 1/3 of isopropyl alcohol (mass fraction) [7.29, 30].
Because the initial focus of the study will concentrate in the mitral position, sensor of the flow meter with ultrasound will be placed in the model of the atrium, measuring the flow before passing through the prosthesis to be studied. The pressure transducers will be arranged before and after the prosthesis and the temperature controller will be installed in the reservoir just before the model of the atrium.
Bench calibration
The first setting on the bench shall be the volume ejected from the ventricle, from physiological data. To do so, it shall be established, via computer and servo drive, the servo drive track path. Initially it will be focused on meeting a ventricular ejection volume of 70 mL, a heartbeat of 70 bpm and a cardiac output of 5.0 L / min. Other adjustments will be set to meet the requirements of ISO 5840:2005 for hydrodynamic testing of prostheses under pulsatile flow.
The measurement of air and fluid volume test in complacency, as well as a pre-adjustment of peripheral resistance via tourniquet will establish initial conditions for placing the bench in operation and, via the acquisition of pressure and flow rate data, adjust it a bit to the curves characteristic of physiological cardiac pulse: a prerequisite for flow measurements in the mitral valve via the LDA system.
Measurements on the bench
At this stage, it is expected to take measurements for characterization of the velocity, pressure and shear stress fields in the prosthetic mitral valves.
Through the academic partnership formally established between the Laboratory of Biomedical and Environmental Engineering (LAB) of the Department of Mechanical Engineering - EPUSP and the Laboratory of Surgical Technique and Experimental Surgery, of the Department of Surgery of UNICAMP, enables the use of the LDA system, whose manufacturer is the company Dantec Dynamics. Such equipment will be allocated on the LAB (PME / EPUSP) coupled to the bench, so as to allow the incidence of laser beams on the prosthesis fixating platform (here it can also be called "optical platform"), thus allowing the instantaneous measurement of the velocity field.
Some preliminary tests should be done in order to allocate and establish the movement of laser beams in the regions of interest: downstream of the mitral prosthesis. Preliminary tests will also be performed in order to collect data from the velocity profiles and correlate them.
Currently, through collaboration established with Braile Biomédica S/A, the biological prosthetic heart valves and pressure transducers produced by this company are now available. Nothing prevents, however, that research leads to new concepts of design and construction of valves, based on hydrodynamic testing.
Computational modeling
The experimental results will be used to adjust a numerical model of CFD (Computational Fluid Dynamics), using the ANSYS (CFX / Fluent), available at LAB (PME / EPUSP). This computer model will be useful to interpret the data of hydrodynamic stress. Also, taking advantage of the experience that has been acquired by forming a study group for learning and training with ANSYS.
Optimization of the prostheses
As seen in the brief literature review, the hydrodynamic stress is an important parameter for characterizing the hemolysis due to the flow in prosthetic heart valves.
The computer model that will be obtained in future works will allow to study, by academic partnership formally established between POLI / USP and the Department of Surgery of UNICAMP, the rates of hemolysis obtained and alleged design optimizations for new mitral valve designed.
ACKNOWLEDGEMENTS
Polytechnic University of São Paulo (EPUSP) and the Department of Mechanical Engineering for the infrastructure support.
Coordination of Improvement of Higher Education Personnel (CAPES) for a PhD scholarship.
REFERENCES
1. Yoganathan AP, Chandran KB, Sotiropoulus F. Flow in prosthetic heart valves: state-of-the-art and future directions, Ann Biomed Eng. 2005;33(12):1689-94. [MedLine]
2. Campos NLKL, Andrade RR, Silva MAM. Anticoagulação oral em portadores de próteses valvares cardíacas mecânicas. Experiência de dez anos. Rev Bras Cir Cardiovasc. 2010;25(4):457-65. [MedLine] View article
3. De Bacco MW, Sartori AP, Sant'Anna JRM, Santos MF, Prates PR, Kalil RAK, et al. Fatores de risco para mortalidade hospitalar no implante de prótese valvar mecânica. Rev Bras Cir Cardiovasc. 2009;24(3):334-40. [MedLine] View article
4. Feguri GR, Macruz H, Bulhões D, Neves A, Castro RM, Fonseca L, et al. Troca valvar aórtica com diferentes próteses. Existem diferenças nos resultados da fase hospitalar? Rev Bras Cir Cardiovasc. 2008;23(4):534-41 [MedLine] View article
5. Lemos PCP, Stolf NAG. A prótese valvar cardíaca definitiva: meio século de procura, Arq Bras Cardiol. 1992;58(1):15-24. [MedLine]
6. Fung YC. Biomechanics: circulation. 2nd ed. New York: Springer;1997. p.577.
7. Berger SA, Goldsmith W, Lewis ER. An introduction to bioengineering. New York:Oxford University Press;1996. p.133-70.
8. Yoganathan AP, He Z, Casey Jones S. Fluid mechanics of heart valves. Ann Rev Biomed Eng. 2004;6:331-62.
9. Dasi LP, Simon HA, Sucosky P, Yoganathan AP. Fluid mechanics of artificial heart valves, Clin Exp Pharmacol Physiol. 2009;36(2):225-37. [MedLine]
10. Meyer RS, Deutsch S, Bachmann CB, Tarbell JM. Laser Doppler velocimetry and flow visualization studies in the regurgitant leakage flow region of three mechanical mitral valves. Artif Organs. 2001;25(4):292-9. [MedLine]
11. De Paulis R, Schmitz C, Scaffa R, Nardi P, Chiariello L, Reul H. In vitro evaluation of aortic valve prosthesis in a novel valved conduit with pseudosinuses of Valsalva. J Thorac Cardiovasc Surg. 2005;130(4):1016-21. [MedLine]
12. Grigioni M, Daniele C, D'Avenio G, Morbiducci U, Del Gaudio C, Abbate M, Di Meo D. Innovative technologies for the assessment of cardovascular medical devices: state-of-the-art techniques for artificial heart valve testing. Expert Rev Med Devices. 2004;1(1):81-93. [MedLine]
13. Milo S, Rambod E, Gutfinger C, Gharib M. Mitral mechanical heart valves: in vitro studies of their closure, vortex and microbubble formation with possible medical implications. Eur J Cardiothorac Surg. 2003;24(3):364-70. [MedLine]
14. Chew YT, Chew TC, Low HT, Lim WL. Techniques in the determination of the flow effectiveness of prosthetic heart valves. In: Cardiovascular techniques: biomechanical systems: techniques and applications. vol. II. London:Cornelius Leondes, CRC Press LLC;2001. p.70-117.
15. FDA U.S. Food and Drug Administration. Draft guidance for industry and FDA Staff. Heart valves - investigational device exemption (IDE) and premarket approval (PMA) applications. January, 2010. Available from: URL: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm193096.htm.
16. Dasi LP, Ge L, Simon HA, SotiropouloS F, Yoganathan AP. Vorticity dynamics of a bileaflet mechanical heart valve in an axisymmetric aorta. Phys Fluids. 2007;19(6):067105-17.
17. Meyer RS, Deutsch S, Maymir JC, Geselowitz DB, Tarbell JM. Three-component laser Doppler velocimetry measurements in the regurgitant flow region of a Björk-Shiley monostrut mitral valve. Ann Biomed Eng. 1997;25(6):1081-91. [MedLine]
18. Woo YR, Yoganathan AP. Pulsatile flow velocity and shear stress measurements on the St. Jude bileaflet valve prosthesis. Scand J Thorac Cardiovasc Surg. 1986;20(1):15-28. [MedLine]
19. Pinotti M. Is there correlation between the turbulent eddies size and mechanical hemolysis? J Braz Soc Mech Sci. 2000;22(4). Available from: URL: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0100-73862000000400006.
20. Pio F. em DCI (Indústria), 19 de abril de 2002. Available from URL: http://www.bv.fapesp.br/namidia/noticia/14726/valvula-cardiaca-mercado-us-10/.
21. Ortiz JP, Bessa KL, Legendre DF, Fonseca J, Cherniauskas R, Andrade A. Physical modeling of the cardiac pump: preliminaries results. In: PCH Notícias & SHP News. 2009;11(42):14-8.
22. Fonseca J, Andrade A, Nicolosi DE, Biscegli JF, Legendre D, Bock E, et al. A new technique to control brushless motor for blood pump application. Artif Organs. 2008;32(4):355-9. [MedLine]
23. Leal EB, Ortiz JP, Guerino SD. Hydrodynamic simulator for studies in vitro of the cardiovascular system. In: 17st COBEM; São Paulo. Proceedings of COBEM 2003. São Paulo:ABCM;2003.
24. Leal EB. Simulador hidrodinâmico para estudos "in vitro" do sistema cardiovascular [Dissertação de mestrado]. São Paulo:Universidade de São Paulo, Escola Politécnica;2001. 84p.
25. American National Standard. Cardiovascular implants - cardiac valve prostheses, ISO 5840:2005.
26. Arita M, Tono S, Kasegawa H, Umezu M. Multiple purpose simulator using a natural porcine mitral valve. Asian Cardiovasc Thorac Ann. 2004;12(4):350-6. [MedLine]
27. Lu PC, Lai HC, Liu JS. A reevaluation and discussion on the threshold limit for hemolysis in a turbulent shear flow. J Biomech. 2001;34(10):1361-4. [MedLine]
28. Bessa KL, Ortiz JP. Flow visualization in arteriovenous fistula and aneurysm using computational fluid dynamics. J Visualization. 2009;12:95-107.
29. Legendre DF. Estudo de comportamento de fluxo através de modelo computacional de aneurisma de aorta infra-renal obtido por tomografia [Tese de doutorado]. São Paulo:Universidade de São Paulo, Escola Politécnica;2009. 183p.
30. Cherniauskas R, Ortiz JP. Fluido de trabalho para a simulação experimental de fluxo sanguíneo em aneurisma de aorta abdominal . São Paulo:Universidade de São Paulo, Escola Politécnica;2009. 8p.
Article receive on Thursday, December 9, 2010
 All scientific articles published at www.rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at www.rbccv.org.br are licensed under a Creative Commons license