The objective is to present the results of the application this device in experimental animals unloading only the left ventricle.
Between June 2002 and October 2009, were implanted in 27 calfs with age between 2½ to 4 months and 80 to 100 kg of weight, with general anaesthesia and controled ventilation, by mean of left thoracotomy a cannula in the apex of VE and a lateral anastomose of a GTFE vascular graft tube in the descending portion of the thoracic aorta, both connected to the device implanted below the diaphragm in the subcutaneous (24) and intrathoracic (three). The cardiopulmonary bypass (BP) was used in five calves, and directly introduce the outflow cannula in 22.
During the implant two and in the first hours of the post operative period (PO) three deaths were observed, one related to the device. The survival between the first and the six PO day was found in 17 calves and between day 8 and day 31 (PO) in five all caused by clinical/surgical problems, and related to the device. The hemodynamic impact by the systemic pressure analysis showed 20 to 40 mmHg increase and the laboratory parameters showed lower levels of traumatic impact to the blood and a good biocompatibility.
This kind of research is arduous and complex where at each experiment many problems are indentified in the implantability and in the device, which are sistematic correct, to became device/procedure safe and effective.
Apresentar os resultados da aplicação deste dispositivo em animais de experimentação, promovendo auxílio hemodinâmico apenas ao ventrículo esquerdo (VE).
Entre junho 2002 e outubro 2009, foram implantados em 27 bezerros com idade 2½ a 4 meses e peso 80/100 kg e, por meio de anestesia geral e ventilação controlada e de toracotomia lateral esquerda, era introduzida cânula no ápice do VE e anastomose término/lateral de tubo vascular de politetrafluoretileno (PTFE) com a porção descendente da aorta torácica, ambos interligados ao dispositivo implantado no subcutâneo abaixo do diafragma (24) e intratorácico (três). Em cinco bezerros, o dispositivo foi aplicado com auxílio de circulação extracorpórea (CEC) e, em 22, sem CEC.
Ocorreram dois óbitos durante o implante e três por causas diversas nas primeiras horas de pós-operatório (PO), sendo um relacionado ao dispositivo. A sobrevivência entre o 1º e 6º dia de PO ocorreu em 17 animais e entre o 8º e 31º dia de PO em cinco, com causas determinantes diversas, não só por problemas clínico/cirúrgicos, mas também relacionados ao dispositivo. O impacto hemodinâmico avaliado pela análise da pressão sistêmica mostrou incremento que variou de 20 a 40 mmHg e os dados laboratoriais analisados demonstraram baixos impactos traumáticos à crase sanguínea e boa biocompatibilidade.
Trata-se de pesquisa árdua e complexa onde a cada experimento são identificados problemas não só de implantabilidade, mas também relacionados ao dispositivo, que vão sendo sistematicamente corrigidos, tornando-o cada vez mais seguro e eficaz.
INTRODUCTION
The priority objective of government institutions for promoting research in Brazil is the generation of proprietary technology and the Brazilian Cardiology and Cardiovascular Surgery was always present, providing qualified professional formation, research centers that created and still generate materials and equipment that have been nationally and internationally implemented.
However, when it comes to Artificial Ventricles and Mechanical Circulatory Assistance the situation is not the same. Currently, this modality is a routine clinical practice in Europe and the United States and its application has not been possible in our country, because these devices are not provided by health officials due to its high cost, and yet, national models are still not available in the market.
The surgical treatment of heart failure has advanced degrees in heart transplantation, as the gold standard, with very positive impacts on life expectancy and quality of life in patients with no prospect of survival in the short term. On the other hand, given the scarcity of donors, the loss of candidates in the waiting phase is usually above 50%, according to the Organ Procurement and Distribution of the Health Department of Sao Paulo. If the transplantation teams had these devices and could use them in these patients, it would be possible to rescue and prevent the loss of these candidates around 80% and transplant 80% with satisfactory results.
These devices are supposed to assist the weakened heart to pump blood and can be used as a bridge-totransplantation, which gives patients a chance to safely wait for a compatible donor. Therapy for Recovery is one of its functions, in which the device does not only keep the patient's life in severe conditions, but it also allows the recovery of cardiac function and can be removed subsequently, and finally the device may be used as Destination Therapy, in which their application is made permanently when the patient has contraindications to heart transplantation as immunological incompatibility, chronic infections and advanced age.
The Heart Institute (InCor-FMUSP), developed and concluded an artificial ventricle project having already clinically applied in patients in the waiting phase for donors [1-4], however, it is not available because it is only indiscriminately being applied in cases isolates. Dante Pazzanese Institute of Cardiology (IDPC-SP) also concluded a spiral blood pump project [5,6] and Kubrusly et al. [7] published an article on intraventricular assist device of axial flow, which still was in experimental phase.
Given this reality, the Division of Bioengineering, in association with the Division of Cardiovascular Surgery at Dante Pazzanese Institute of Cardiology (IDPC) developed a prototype of electromechanical operation and pulsatile flow [8-10]. The resulting device is originated from ejection alternating two diaphragms situated in two pumping chambers and its movements performed by brushless motor, controlled by an electronic controller with rechargeable batteries and can be used as an auxiliary to the left ventricle (LV) for both ventricles, even as artificial heart entirely replacing the native organ. In the contractile chamber input and output, two bovine pericardial bioprosthesis were positioned (25 mm and 23 mm) to make the flow become unidirectional, relieving the native ventricular output and providing adequate blood volume which may vary depending on preload alterations or ventricular filling pressure [11-13] (Figures 1 and 2).
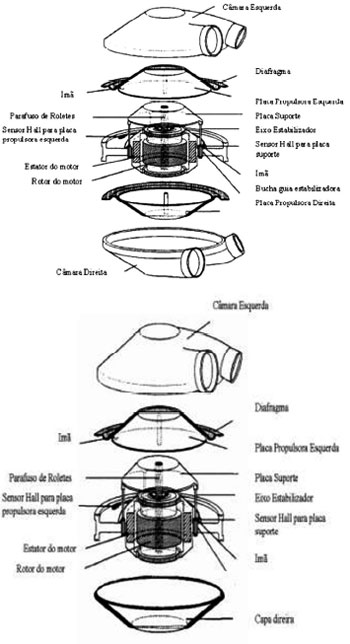
Fig. 1 - Device Settings - (a) Auxiliary Artificial Heart (AAH) (b) Ventricular Assist Device (VAD)
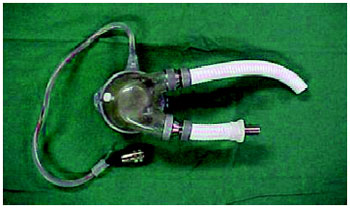
Fig. 2 - Auxiliary Artificial Heart (AAH) configured as a Ventricular Assist Device (VAD)
In order to evaluate the device performance, "in vitro" tests were conducted, in which we aimed to verify the hydrodynamic impact as unicamerality, and "in vivo"tests, where it was implanted to aid the left ventricle (LV) in experimental animals (calves) and monitored their survival [10,14], with the aim to evaluate their performance and the host organism interaction.
METHODS
For "
in vitro" evaluation of the device, closed circulation circuit was used, which allows evaluating the hemodynamic performance, responsiveness to filling identifying possible leaks in the connections, manufacturing defects on the carcass made of acrylic resin, as well as in the polyurethane diaphragm and possible changes for pressure / flow relationship, in another words, flow or output as a function of preload. (Figure 3)
Fig. 3 - Circuit Simulator
This test system was developed by the Department of Surgery at Baylor College of Medicine, in Houston, Texas, to evaluate orthotopic artificial heart development at that institution [12]. The hydrodynamic performance tests were conducted with the pumped flow (cardiac output) being recorded for different left preloads. The preloads were adjusted in increments of 2.5 mmHg in the following values: 0, 2.5, 5, 7.5, 10, 12.5, 15, 17.5 and 20 mmHg. The mean ventricular preload was fixed at 100 mm Hg. AAH operated in VF and FF with frequencies of 80, 100, 120 and 130 bpm.
"In vivo" tests were retrospective experimental trials in which 27 bovine animals were selected from Gir or Gyr breed (¾ or ½) females, aged 4 months and weighing between 85 to 105 kg, without cardiac contractile dysfunction, in which devices were implanted under general anesthesia and controlled ventilation via left lateral thoracotomy, aiming to access its performance, organic alterations and surgical implantability, with survival equal to or greater than 30 days as its main outcome.
It was intended to apply it only as an aid to the left heart, by introducing the tube collector into the cavity of the LV apex and the suture of the outlet tube into the aorta through end-to-side distal anastomoses, initially in the ascending and then in the distal middle portion of the descending aorta. The device's power cord was exteriorized to the skin in a higher and further position from the thoracotomy incision, positioning the device in a specific pocket made in the left, subcutaneous, intradiaphragmatic and subsequently in the left supradiaphragmatic and intrathoracic flanks.
In the first five cases, the device implantation was performed with the aid of EC. However, with the development of sharps device for the LV, seeking direct access to the stainless steel coring device in the left ventricle apex with sharp tips, which introduces titanium cannula connected to the flexible polytetrafluoroethylene (PTFE) tube, thus, extracorporeal circulations was not necessary for its implantations (Figure 4).

Fig. 4 - Apparatus with Sharps Device of LV
Figures 5a, 5b, 5c, 5d and 5e illustrate the surgical time of the device implantation in the experimental animal.
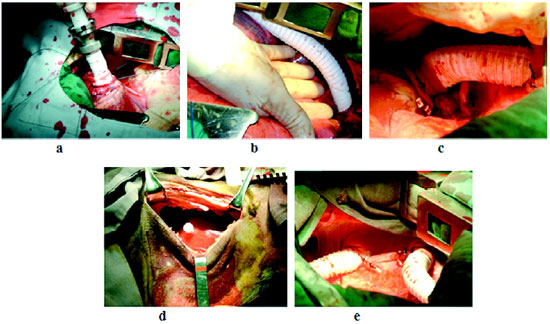
Fig. 5 - (a) Direct introduction of the tube collector into the left ventricle apex via lateral thoracotomy (5th LICS) and its attachment. (b) Endto- side anastomosis of the Gore-Tex tube (outlet) in the distal third portion of the descending thoracic aorta (c) LV apex cannulated in its original position with the outlet tube crossing the diaphragm toward the device below the diaphragm (d) Introduction of device into the attic subdiaphragmatic pouch (e) collector (E) and outlet tube (D) crossing the diaphragm toward the device
Figure 6 shows the scheme of the anatomical disposition of the implant and the experimental animal with the implanted device in the recovery phase.
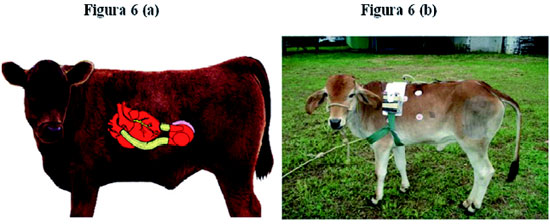
Fig. 6 - Scheme of the anatomical disposition of the implant and the experimental animal with the implanted device in the recovery phase
In cases when extracorporeal circulation was employed, transoperative anticoagulation was consisted of intravenuos heparin application 5mg/kg/ body weight seeking to keep Activated Clotting Time (ACT) between 600 and 800 seconds. In all experiments with or without EC during postoperative (PO) period, it was intended to maintain ACT between 350-450 seconds with intravenous heparin solution. From the fifth/seventh postooperative period on, coumarins were introduced orally, (Marcoumar, or Marevan Cumadin) maintaining prothombin time (PT) between 20 and 40% and INR between 3 and 3.5 (15). In the second postoperative period, if the animal was normally eating standing up, it would be transferred to anti-slip platform with side supports. The pressure data access were kept and evaluated for 7 days and continuous electrocardiogram (ECG) was monitored throughout the experiment.
From the laboratory point of view, the following parameters were evaluated: arterial/venous gasometry, potassium, sodium, hemoglobin (Hb), hematocrit (Hct), leukocytes, platelets, urea, creatinine, glucose, AST, gamma GT, prothrombin time, INR , TCA were performed in all experiments, however, they were thoroughly analyzed in only five animals whose survival rates were the highest ones (7 to 31 days).
This is a prospective cohort study that underwent numerous changes throughout the research and, due to this fact, a more elaborated data application was not possible, as well as the sample choice.
RESULTS
"In Vitro" Tests
Hydrodynamic performance was evaluated by monitoring the output as a function of the left preload. When the device was operating at variable frequency (VF), its elevation caused by increased left preload has shown it has high sensitivity to filling pressure, similar to Frank- Starling effect. The maximum cardiac output of 6 L/min was obtained by VF, operating around 200 bpm, under a left filling pressure of 20 mmHg. When the pressure remained normal (10 mmHg) frequency was 180 bpm, providing a cardiac output of 5 L/min against a mean afterload of 100 mm Hg. At a fixed frequency (FF), especially for pre-loading above 2.5 mmHg, the output was not affected, due to the fact that it is necessary low pressure to achieve complete filling of the chamber for adjusted frequencies, it means that when the pre-loaded is higher than 2.5 mmHg and frequency between 80 to 130 bpm, the device operates completely filled [9] (Figure 7).
Fig. 7 - Performance Curve (Flow in L / min X mmHg preload) operating at variable frequency (VF) and fixed frequency (FF) at 80, 100, 120 and 130 bpm
Between June 2002 and November 2009, this test was performed in 27 calves, according to Tables 1 and 2, in which whether the EC was used or not the device implantation, survival and the causes behind the occurrence of the animal death. The experiments with survival up to 6 days and those who survived of 7 to 31 days were selected.
In experiments that showed increased survival (7 to 31 days) the following laboratory data were determined: hemoglobin, hematocrit, leukocytes, platelets, glucose, fibrinogen, urea, creatinine, AST, Gamma GT, TCA (1st PO period), TCA, PT and INR, with the aim to analyze its traumatic impact to the blood crasis.
Statistical data analysis was performed by ANOVA, taking into account the +mean values, standard deviation, fast opening and closing for the significance calculation (
P = 0.05) compared to one another based on the survival time as a variable (Figure 8a-n). Normal values +are determined by dotted lines in the charts.
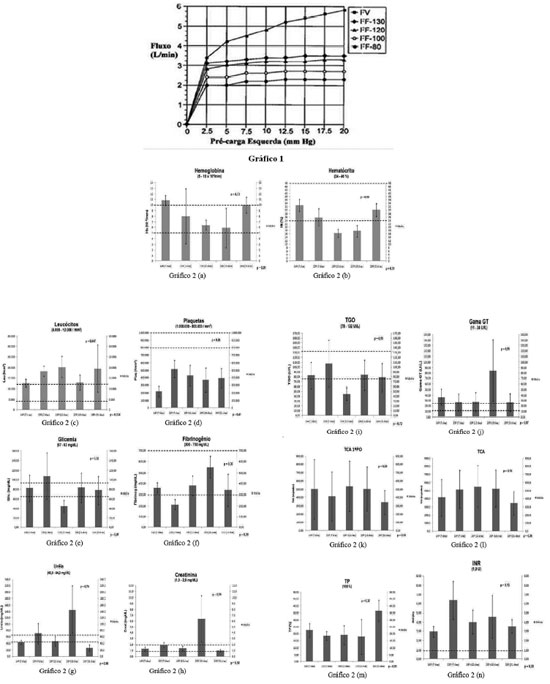
Figure 8 - Statistical analysis taking into account the +mean, standard deviation, fast opening and closing values for significance calculation
The platelet count was lower than initial values (the N/ 800.000 1.000.000/mm3) in all animals, but with no statistical difference among them, and there is only one parameter, leucocytes, that showed differences with statistical significance (
P = 0.047 and 0.034). The evaluation of anticoagulation data showed appropriate values +for the procedure. The others showed no significant changes, proposing that the impact of the device showed no deterioration proportional to the time of application, presuming a small traumatic impact for the blood crasis.
DISCUSSION
The conception of a mechanical circulatory assist device should take into account many variables in order to obtain good results and performance, and its design should be consistent in generating a minimum traumatic flow, with no areas that could cause damage to blood cells. The structure must be solid and resistant to temperature and wear, without tissue toxicity levels. It must be safe, functional and have anatomical (avoiding mechanical compressions in tissues or vital organs), blood (avoiding harm to the elements and pathological stimuli to the circulation system) and tissue biocompatibility (avoiding thermal, electrical and toxic damages to tissues) [15]. These are variables that make these types of research complex and arduous, requiring a lot of tenacity and commitment, especially when there is no prior experience of the subject.
Many modifications were not only implemented in the device and its components [16,17,19,20], but also in the surgical technique for implantation, based on experience acquired in each experiment. The circuit simulator for "in vitro" test was changed by adding connections for pressure evaluation performed +by a multi-parameter monitor, flow meter, circulationg fluid heating system at 37 º C with a thermometer and a stainless steel reservoir to accommodate accessories, making it more reliable before its application in experimental animals.
Several changes related to its internal and external format were introduced. The metal connections and the stainless steel cannula of the LV apex were exchanged for titanium 2, with a proven better histocompatibility, mechanical strength and corrosion, as well as lower modulus of elasticity and, structural changes in shape and finishing, facilitating its implantation and performance.
The tubes that make the connections between the native heart / aorta with the device, initially made of silicone, were replaced by polyester (DACRON) and later by structured PTFE (Goretex), with higher hemocompatibility and better surgical handling.
The assembly of electronic components was also improved, as the use of electric wires and cables with no correction or welds, which eventually led to ruptures and consequent malfunction. The coupling, initially with threads of the electronic connectors of the power cord as the controller, went into fast mode, including moisture sealing. The electrical power cord, initially consisted of two cables became single, including the contractile chamber air-hole and its thicker and more resistant silicone coating to avoid solution of continuity. Initially employed, the lead-acid battery has been replaced by a smaller nickel-metal hydride (NiMH) battery, with a smart recharging system and also with LED information on load and duration.
The firing system in its first version was powered by using an electronic assembly and a microcomputer using a software program in C language if necessary, therefore, besides the fire motor plate, there was a PC connection. In the second version, this component was replaced by a standalone unit, which was able to control the device without a connection to the PC. However, it has been preserved in order to record parameters such as filling time, ejection and firing frequency. Aiming to reduce its dimensions, but following the previous release technology (micro-controlled system), surface mount components (SMO) started being used, enabling not only the size reduction, but also the incorporation of wireless telemetry with remote monitoring [21].
The surgical technique for implantation has also evolved, making the procedure simpler. Initially, in order to position the cannula of the LV apex via the left lateral thoracotomy (4th LICS), EC would be used and distal anastomosis of the tube performed + in the ascending aorta. As the sharps device has developed, which incorporates around the cannula connected to all pre PTFE, its introduction into the LV was feasible without the aid of EC and distal anastomosis of the tube had to be performed in the middle third portion of the descending thoracic aorta, making the implant quicker and safer.
The device pocket was initially made +through another incision in the subcostal left flank and after its placement; the inlet and outlet tubes were obligated to cross the diaphragm muscle, which makes it an important cause of morbidity in the evolution of the postimplant animal. More recently, through thoracotomy in the 5th LICS, the device was positioned in the left pleural cavity in space obtained by dissection above the diaphragm toward the abdomen without angulation of the inlet and outlet tube, preserving the integrity of the diaphragm and avoiding another surgical incision.
Other aspects that involved with the physical, personnel and logistics infrastructure have been improved with the creation Research Center for Assisted Circulation at IDPC, with operating rooms, ICU, veterinary medical team monitoring pre-and postoperative period, careful selection of animals with early assessments from clinical / laboratory parameters, which will optimize this research results, gathering evidence to consolidate its application in humans.
This present article is in experimental phase and is open to criticism, since its initial protocol with pre-established methodology was modified according to the findings and experience acquired to each experiment. Another aspect to be criticized is the application of mechanical circulatory assist device in experimental animals without clinical evidence of myocardial contractile failure and, therefore, it is not possible to evaluate its hemodynamic impact on the host organism, observing only a slight increase in systemic blood pressure in the first moments after implantation, taking into account the subsequent circulatory readaptation. The evidence of hemodynamic impacts was obtained in
"In Vitro" tests, where it was possible to accurately assess their performance. The absence of more detailed investigation of their impacts on the circulation, invariably present when interposing elements in the bloodstream [22], causes hemolytic and inflammatory trauma with increased serum/urinary levels of free hemoglobin and even hemolysis. When analyzing the chart 5d (Platelets) a count below usual was observed, however, with no statistical differences between values +(
P = 0.26) and survival time variable (
P = 0.41). The same fact was noticed in relation to the Fibrinogen
P = 0.30 and 0.39 respectively, suggesting a possible low-impact trauma.
After all, it is possible to state that this research has led us to consistent and intense learning, enabling us to plan prospective experimental study with strict protocol, searching for evidence that could substantiate their application in human patients that meet the indications for mechanical circulatory assistance for clinical cases of severe myocardial contractile failure.
REFERENCES
1. Leirner AA. Assistência mecânica no tratamento de insuficiência cardíaca grave. Projeto, construção e teste de um ventrículo artificial [Tese de Livre Docência]. São Paulo:Faculdade de Medicina da Universidade de São Paulo;1995.
2. Oshiro MS, Hayashida SA, Maizato MJ, Marques EF, Stolf NA, Jatene AD, et al. Design, manufaturing and testing of a paracorporeal pulsatile ventricular assist device: São Paulo Heart Institute VAD. Artif Organs. 1995;19(3):274-9.
3. Benício A, Moreira LFP, Hayashida S, Cestari IA, Leirner AA, Stolf NAG, et al. Avaliação do desempenho hemodinâmico do dispositivo de assistência ventricular InCor como substituto do coração esquerdo. Rev Bras Cir Cardiovasc. 1999;14(3):237-46.
4. Galantier J. Avaliação de emprego clínico do dispositivo de assistência ventricular INCOR como ponte para transplante cardíaco [Tese de Doutorado]. São Paulo:Faculdade de Medicina da Universidade de São Paulo;2007.
5. Dinkhuysen JJ. Bomba superfície espiral: concepção, desenvolvimento e aplicação clínica de projeto original [Tese de Livre Docência]. São Paulo:Faculdade de Medicina da Universidade de São Paulo;2005.
6. Dinkhuysen JJ, Andrade AJP, Manrique R, Saito CSM, Leme J, Biscegli JF. Bomba sanguínea espiral: concepção, desenvolvimento e aplicação clínica de projeto original. Rev Bras Cir Cardiovasc. 2007;22(2):224-34.
7. Kubrusly LF, Martins AF, Madeira J, Savytzky S, Wollman D, Melhem A, et al. Dispositivo de assistência circulação mecânica intraventricular de fluxo axial: estudo in vitro. Rev Bras Cir Cardiovasc. 2000;15(2):169-72.
8. Andrade AJP, Nicolosi DEC, Lucchi JC, Biscegli JF, Arruda ACF, Dhashi Y, et al. Auxiliary total artificial heart: a compact electromechanical artificial heart working simultaneously with the natural heart. Artif Organs. 1999;23(9):876-80.
9. Andrade A, Ohashi Y, Lucchi J, Nicolosi D, Dinkhuysen JJ, Biscegli J, et al. Testes "in vitro" e "in vivo" com o coração artificial auxiliar (CAA): um novo modelo de coração artificial totalmente implantável e heterotópico. Rev Bras Cir Cardiovasc. 1999;14(2):128-34.
10. Andrade AJP, Ohashi Y, Lucchi JC, Nicolosi DEC, Dinkhuyden JJ, Biscegli JF, et al. in vivo test with eletromechanical auxiliary total artificial heart (ATAH). ASAIO J. 1999;45:171.
11. Legendre DF. Estudos de técnicas de texturização e biolização e desempenho biológico "in vitro e "in vivo" em membrana para um dispositivo de assistência ventricular e coração artificial totalmente implantável [Tese de Mestrado]. São Carlos: Universidade de São Paulo;2003.
12. Orime Y, Takatani S, Tasai K, Ohara Y, Naito K, Mizuguchi K, et al. in vitro and in vivo validation tests for total artificial heart. Artif Organs. 1994;18(1):54-72.
13. Legendre D, Silva OL, Andrade A, Fonseca J, Nicolosi D, Biscegli J. Endurance test on a textured diaphragm for the auxiliary total artificial heart (ATAH). Artif Organs. 2003;27(5):457-60.
14. Fonseca JWG, Andrade AJP, Bock EGP, Leme J, Dinkhuysen J, Paulista PP, et al. "in vivo" tests with the auxiliary total artificial heart as a left ventricular assist device in calves. ASAIO J. 2005;1(1):1-5.
15. Andrade AJP, Nicolosi DEC, Biscegli JF, Bock EGP, Fonseca JWG. Blood coagulation time control, during in vivo tests with the Auxiliary Total Artificial Heart (ATAH) implanted in calves as left ventricle assist device (LAVD). ASAIO J. 2006;52(2):63A.
16. Antaki JF, Poirier V, Pagani FD. Engineering concepts in the design of mechanical circulatory support. In: Frazier OH, Kirklin JK, eds. ISHLT Monograph Series. Vol. 1. 2006. p.33-52.
17. Ohashi Y, Andrade A, Müller J, Nosé Y. Control system modification of an electromechanical pulsatile total artificial heart. Artif Organs. 1997;21(12):1308-11.
18. Nakata K, Ohashi Y, Muller J, Andrade AJP, Nosé Y. Simplified control mechanism for electromechanical total artificial heart (TAH) - in vitro study. Artif Organs. 1997;21:514.
19. Ohashi Y, Andrade AJP, Muller J, Nosé Y. Augmented destruction test with an electromechanical pulsatile total artificial heart. Artif Organs. 1999;23(9):884-7.
20. Andrade AJP, Barbosa MP, Nicolosi DEC, Fonseca JWG, Biscegli JF, Arruda ACF, et al. Improvement on the auxiliary total artificial heart (ATAH) left chamber design. Artif Organs. 2003;27(5):452-6.
21. Bronzieri R, Nicolosi DEC, Fonseca JWG, Andrade AJP, Lucchi JC, Bock EGP, et al. An implantable electronic system with fuzzy control used in auxiliary heart. ASAIO J. 2006;5(2):62A.
22. Ohashi Y, Andrade A, Nosé Y. Hemolysis in an electromechanical driver pulsatile total artificial heart. Artif Organs. 2003;27(12):1089-93.








 All scientific articles published at www.rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at www.rbccv.org.br are licensed under a Creative Commons license