This study aims to evaluate the effects of prophylactic heart donor tricuspid annuloplasty in patients after heart transplantation with bicaval anastomosis.
From 2002 to 2005, 20 patients undergoing heart transplantation with bicaval anastomosis and with a survival rate over 6 months were deliberately selected. Patients were divided into two groups: Group I - 10 patients who underwent prophylactic heart donor tricuspid annuloplasty by the De Vega technique; and Group II - 10 patients did not undergo annuloplasty. In both groups, presurgical clinical characteristics were the same. The tricuspid regurgitation degree was evaluated by transthoracic Doppler echocardiography and it was qualified from 0 to 3 (0=absent, 1=mild, 2=moderated, 3=severe). Myocardial performance was evaluated by ventricular ejection fraction and invasive hemodynamic study performed during routine endomyocardial biopsies.
Mean clinical follow-up was 14.6±4.3 (6 and 16) months. There was only one death in group II. It was not related to annuloplasty. Mean degree of tricuspid regurgitation in Group I was 0.4±0.6 and in Group II was 1.6±0.8 (p < 0.05). There was a statistically significant difference between both groups in right atrium pressure, which was higher in Group II.
In view of the limitations of the study, the prophylactic tricuspid annuloplasty in heart donor reduced the degree of valvar regurgitation in the medium term after heart transplantation with bicaval anastomosis, in spite of not interfering with the allograft hemodynamic performance in the period under consideration.
O presente estudo tem por objetivo avaliar os efeitos da anuloplastia tricúspide profilática no coração doador em transplante cardíaco com anastomose bicaval.
De 2002 a 2005, foram selecionados de forma não aleatória 20 pacientes submetidos ao transplante cardíaco pela técnica bicaval e com sobrevida superior a seis meses. Eles foram divididos em dois grupos: Grupo I - 10 pacientes que receberam coração doador com anuloplastia tricúspide profilática, pela técnica de De Vega; e Grupo II - 10 pacientes que não receberam a anuloplastia, ambos com características semelhantes. O grau de regurgitação tricúspide foi avaliado pela ecocardiografia transtorácica com Doppler e foi quantificado entre 0 e 3 (0=ausente, 1=discreto, 2=moderado, 3=grave). O desempenho miocárdico foi avaliado pela fração de ejeção ventricular e pelo estudo hemodinâmico invasivo, durante as biópsias endomiocárdicas de rotina.
O período médio de observação foi de 14,6±4,3 meses (6 e 16 meses). Houve apenas um óbito no grupo II não relacionado à anuloplastia. O grau médio de regurgitação tricúspide no Grupo I foi de 0,4±0,6 e no Grupo II foi de 1,6±0,8 (p < 0,05). Dentre as variáveis analisadas, houve apenas diferença estatisticamente significativa na pressão do átrio direito do Grupo II, que foi maior.
Respeitando-se as limitações do estudo, pode-se observar que a anuloplastia tricúspide no coração doador reduz a regurgitação em médio prazo após o transplante cardíaco pela técnica bicaval, a despeito de não interferir no desempenho hemodinâmico do enxerto, no período considerado.
INTRODUCTION
Tricuspid insufficiency frequently occurs after orthotopic heart transplantation, and with clinically variable manifestations [1,2]. The most severe presentations, which have exuberant clinical expression of right cardiac insufficiency with the need for surgical intervention into the tricuspid valve (plastic repair or replacement), appear more rarely [3]. Different factors contribute to the pathophysiological mechanisms that participate in tricuspid regurgitation. The non-identified primary valvular lesion is rare, since the donor heart is largely investigated before use and, in the case of this lesion identification, the heart can be rejected, or a prophylactic correction can be performed [3]. The secondary dysfunction is the most common situation, and it is fundamentally caused by either annular dilatation that predisposes to valve prolapse or by chordae tendinae lesion originating from multiple routinely performed endomyocardial biopsies [4]. Generally, both types of valvular lesions previously mentioned are present and contribute, in variable grades, to the tricuspid insufficiency.
Apart from the multifactorial aspect that involves it, the lesion on tricuspid valve occurs more frequently in transplants that use classical technique [5] than in the transplants using the bicaval technique [6]. Anastomosis, as proposed by Lower et al. [5], creates enlarged atrial cavities with abnormal geometry through the fusion of the atrium stumps of donor and recipient. The enlarged atrial cavities lose their proportionality and modify the tricuspid ring movement, which is one of the causes of valvular regurgitation after transplant [7]. Other factors that significantly contribute are pulmonary resistance from the recipient, improper myocardial preservation (especially of the right ventricle), lesions of reperfusion, rejection, drastic differences between donor weight and recipient weight, and lesions on anatomic structures of the valve apparatus made during the endomyocardial biopsies [1,4].
In a cardiac transplant, a tricuspid insufficiency can produce severe consequences, such as a delay in graft recovery, predisposition to appearance of right ventricle dysfunction (followed by clear right cardiac failure), and reduction of the graft or patient survival in the long-term [6,8]. The tricuspid valve, for the reasons described above, is the most vulnerable and, thus, suffers the most from heart transplantation (followed by mitral and aortic valves) [9,10].
The tricuspid annuloplasty is largely used for isolated tricuspid insufficiency repair, or when associated to other valve lesions, and is more frequent in mitral valve involvement [7]. Currently, there is no evidence that demonstrates its real benefit when performed in the donor heart as a preventive method against postoperative valvular insufficiency [11,12]. However, initial experiments with the use of prophylactic annuloplasty in the tricuspid valve performed in isolated transplant centers indicate hemodynamic advantages, especially in cases that used the classical technique. More recently, the experiments have also indicated an increase in the chances of the patient's survival [11,13].
Considering the facts above, the present investigation aims to evaluate the late hemodynamic effects of prophylactic tricuspid annuloplasty in the donor heart in patients that underwent orthotopic heart transplantation with bicaval anastomosis.
METHODS
Since 1985, 343 patients have undergone heart transplantation in the Heart Institute of the Institute of Medicine of the University of São Paulo (InCor). In January 2002, prophylactic tricuspid valve annuloplasty began being used in predetermined nonrandomized cases. For this investigation, 20 patients with survival over 12 months were selected. The study was restricted to the 18th postoperative month. For comparative analysis, the patients were divided into the following two groups:
Group I - composed of 10 patients whose donor heart received tricuspid annuloplasty by De Vega technique: Group II - composed of 10 patients whose donor heart did not receive annuloplasty.
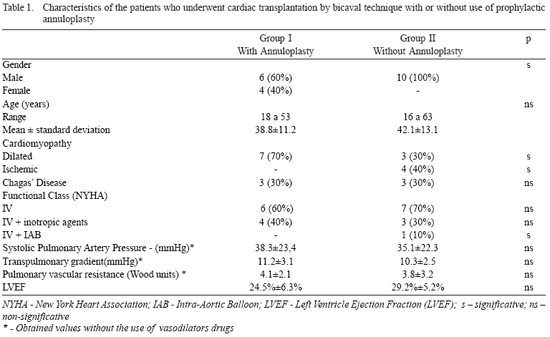
The studied variables were collected prospectively in a specific database, and were retrospectively analyzed. The preoperative clinical characteristics of the selected patients in both groups are described in Table 1.
For donor heart cardioprotection, St. Thomas' II crystalloid cardioplegia solution was applied. It was administered in the ascending aorta during cardiectomy, and immersed in cold saline solution for transportation. During the implantation, cold anterograde cardioplegia was applied, and was repeated every 20 minutes. The adopted operation technique for transplantation in both groups followed the principles developed by Sarsam et al. [14], in 1993, and Aziz et al. [6], in 2002.
During the study period, one surgeon was responsible for the graft removal, and two others were responsible for organ transplantation. The tricuspid annuloplasty applied to the patients of Group I was always performed externally during the donor heart preparation before implantation. For standardization reasons, it was performed by only one surgeon. The tricuspid valve was observed through the orifice of the inferior vena cava. Its ring was reduced to a size of 29mm with the assistance of a 2-0-gauge polyester thread, in double suture, passing through the tricuspid valve ring that is adjacent to anterior and posterior cusps, from the anteroseptal up to posteroseptal commissure, and supported by Teflon pillows, as shown in Figure 1.
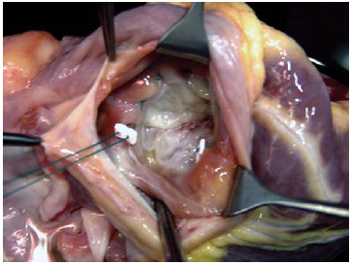
Fig. 1 - In the donor heart, the tricuspid valve can be observed through the orifice related to the inferior vena cava inlet. The annuloplasty by De Vega technique begins with the passage of the threads through the anteroseptal commissure, and it ends at the posteroseptal commissure, passing through the anterior commissure ring of the tricuspid valve.
In the postoperative, the immunosuppression protocol for patients with Chagas' disease was composed of azathioprine, corticoid and cyclosporine [15]. For the others, either Azathioprine or a combination of Mycophenolate mofetil, corticoid and cyclosporine was used. To control rejection episodes, Gallium mapping or endomyocardial biopsy was used, and the degree of rejection was quantified according to criteria adopted by the International Society for Heart and Lung Transplantation [16].
The clinical variables considered for the surviving patients' follow-ups were: functional class of cardiac insufficiency, according to the New York Heart Association (NYHA); evolutionary echocardiographic parameters, and an invasive hemodynamic study, which was performed during routine endomyocardial biopsies. The quantitative variables were considered for up to one year of evolution after transplantation.
The parameters that were elected in the transthoracic Doppler echocardiograph for analysis were the left ventricle ejection fraction (LVEF) determined by the Simpson method, and the tricuspid valve behavior, in which the regurgitation degree was quantified as: 0=absent, 1=mild, 2=moderate and 3=severe, according to proposed by Mugge et al., in 1990 [17].
The direct catheterization routinely performed during endomyocardial biopsies was performed with the assistance of Swan-Ganz catheter, determining the following variables: right ventricle pressure, right ventricle, pulmonary artery and cardiac debit.
The hemodynamic and echocardiographic values, determined by histopathological analysis or clinical presentation rejection, required the use of pulse therapy. This action aimed to exclude possible rejection interference in the studied parameters.
The study herein presents the following limitations: the investigation was performed using single-center, small casuistic, nonrandomized research; and the study used prospective data collection and retrospective analysis.
Statistical analysis
The continuous variables were expressed as mean values and standard deviation. The non-matched Student t-test was used for comparative analysis of the patient groups. The variable categories were expressed frequently and the chi-square test was used to compare the groups. P-value less than 0.05 was chosen for significance.
RESULTS
The mean times of cardiopulmonary bypass, aortic occlusion and graft anoxia in both groups were, respectively: 142.2±20.3 minutes; 86.2±14.6 minutes and 165.1±20.2 minutes in Group I; and 131.5±18.6 minutes, 86.5±17.3 minutes and 158.5±23.7 minutes in Group II (p>0.05). All patients received inotropic support during the operation, as well as postoperative with dobutamine, dopamine, adrenalin, noradrenalin, milrinone, or other associations.
The mean time of intensive care unit (ICU) stay and hospital admission in both groups were: 13.5±3.2 days and 27.2±4.1 days in Group I; and 18.4±5.6 days and 36.2±6.3 days in Group II (p>0.05).
The mean time of postoperative follow-up in Group I was 15.6±2.8 months. In Group II, it was 16.8±2.7 months (p>0.05).
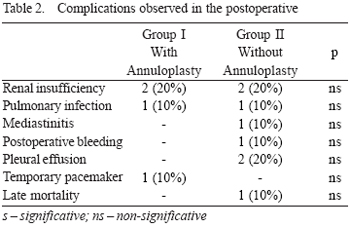
In the mean period of the study, only one (10%) death occurred (p>0.05) in Group II, caused by sudden ventricular tachyarrhythmia of unknown cause in the 8th postoperative month. The main complications observed in postoperative in both groups in the period considered are shown in Table 2.
The mean values of left ventricle ejection fraction determined by echocardiography in Groups I and II were, respectively: 63.3%±8.9% and 70.1%±9.3% (p>0.05). Two patients (20%) from Group I and five (50%) from Group II presented significant right ventricle dysfunction in the immediate postoperative, which was reverted with the use of inotropic agents and vasodilators (p<0.05).
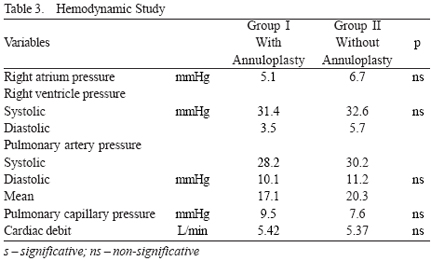
In Group I, the median degree of tricuspid regurgitation was 0.4±0.6 (between 0 and 2). In Group II, it was 1.6±0.8 (between 1 and 3) (p<0.05). The hemodynamic parameters observed in both groups are shown in Table 3.
DISCUSSION
The tricuspid insufficiency is the most common valvular dysfunction after orthotopic heart transplantation [1]. It occurs in a variety of ways, and may reach numbers of approximately 34% [1,2,6]. Valvular involvement presents progressive aspect over time, affecting about 7.8% of the patients in the fifth year after transplantation and occurring in 14.2% by the tenth year [2,4,6]. Similar outcomes were observed in InCor's research, and were presented in a previous publication [18]. The absence of uniformity in the tricuspid insufficiency quantification and the varied distribution of factors that contribute to its evolution are responsible for the occurrence of the dispersed values.
The clinical manifestations of tricuspid insufficiency after transplantation are commonly benign, and few of them require surgical treatment. The progressive aspect of tricuspid insufficiency may determine long-term appearance of ascites and edema of the lower limbs. It can lead to or aggravate varied degrees of renal insufficiency, can increase the occurance of right ventricle dysfunction, can compromise graft recovery, can increase the postoperative morbidity, and can compromise graft or patient survival [1,2,4,19].
The outcomes indicate advantages of the bicaval technique over the classical technique in terms of tricuspid insufficiency prevention, since there is a higher possibility of distortion among the new atrial cavities and the ventricles [6,8,9]. The anatomic graft distortion mainly modifies the tricuspid ring geometry, which, when associated with other factors (such as the pulmonary hypertension), provides fertile ground for development of valvular insufficiency. This same phenomenon also occurs in the mitral ring; however, in a less pronounced manner due to anatomical differences between the left ventricle and the right ventricle.
In other publications, Aziz et al [6] emphasize that the bicaval anastamososis was not able to definitively remove the tricuspid insufficiency after orthotopic transplantation; however, it has reduced its occurrence from 50% in the classical technique to 19.9% in the bicaval technique. Similar behavior was observed by Park et al. [21] in 2005, when they compared two different groups of patients who received heart transplantation. On that occasion, they noticed the presence of tricuspid insufficiency in 36.4% and 10.5% from classical and bicaval techniques, respectively.
When tricuspid regurgitation is severe and with exuberant clinical expressions of right failure, surgical treatment with annuloplasty or valve replacement with prosthesis are indicated [3]. In 2006, Filsoufi et al. [22] verified that 5.8% of patients who underwent heart transplantation developed significant tricuspid insufficiency and needed surgical correction after 21 postoperative months of evolution. The annular dilatation was responsible for half of the cases with tricuspid regurgitation. As for the InCor experience, only one patient (1/343; 0.3%, and who did not participate in the study) needed late tricuspid valve replacement due to severe and progressive insufficiency after transplantation by classical technique.
The chordae tendinae lesions from repeated endomyocardial biopsies represent anther frequent cause of tricuspid valve insufficiency and may reach numbers up to 47% [1,4,23]. On the other hand, the tricuspid insufficiency manifests by primary graft dysfunction or primary valvular lesion, and offers serious midterm and longterm consequences to the patient [19]. In the present study, the retrospective analysis of the anatomicopathological examinations did not identify the presence of chordae tendinae fragments in any of the slides. Possibly, this low incidence of traumatic tricuspid lesions observed during the study is fundamentally due to two factors: the use of Gallium mapping, which significantly reduced the biopsies, and the restricted number of surgeons who have had extensive practice with the procedure.
Even though the bicaval technique has reduced the occurrence of tricuspid insufficiency, its outcomes are still controversially presented [6,8-10]. Thus, the prophylactic tricuspid annuloplasty was introduced as an alternative method to assist in the prevention of secondary valvular dysfunction caused by ring dilatation, despite the inability to avoid the iatrogenic lesions in the chordae or in the cusps [11,13]. In 2004, Jeevanandam et al. [11] presented a randomized, comparative study with one year of observation about the prophylactic application of the annuloplasty using the De Vega technique in the donor heart in patients who received heart transplantation with bicaval anastomosis. The authors concluded that the prophylactic application has improved the immediate function of the graft, with better right ventricle performance without an increase in immediate mortality or anoxic time. More recently, they observed interference in the increase in survival of those patients who received the tricuspid annuloplasty in the donor heart [13].
In 2004, Brown et al. [12] also reported an experience with 25 consecutive cases of patients who underwent orthotopic heart transplantation using the classical technique, during which an annuloplasty was applied using either the De Vega technique or the ring implant. Similarly, they observed a significant reduction of the tricuspid insufficiency without morbidity increase. Considering that the authors used only the classical technique, we can predict higher expressivity in the determination of valvular competency obtained after transplantation.
The present study was motivated by annuloplasty benefits indicated in the literature, as mentioned above, and also because it may represent a significant technical advance in the evolution of transplants without an additional increase in morbidity. The exclusion of the patients who received transplant by classical technique did not aim to produce a bias that could interfere in the outcomes analysis.
Patients who present pressure or high pulmonary vascular resistance (PVR) after transplantation present higher risk for appearance of tricuspid insufficiency caused by right ventricle dilatation and, consequently, by the tricuspid ring as well. The pulmonary hypertension alone is well-tolerated in the presence of preserved right ventricular function. However, the superposition of these factors contribute quite injuriously to the right ventricle and to the appearance of tricuspid insufficiency. The right dysfunction may arise from a poor myocardial perfusion, reperfusion injuries, air embolism, weight incompatibility between donor and recipient, rejection, or a combination of these factors [24].
The patients of this present investigation did not present high pressure in the pulmonary artery and also presented hypertension in the postoperative, which may have contributed to the reduction of possible annuloplasty benefits. It also can be observed that the right ventricle dysfunction was present in 20% of the patients who received prophylactic annuloplasty, whereas, in those who did not receive prophylactic annuloplasty, the rate of occurrence was 50%.
The valve repair using the De Veja technique is well established, and it is largely used in isolated tricuspid insufficiency repair or associated to other valvular lesions - specifically the mitral valve- with good long-term outcomes [7]. It deals with a simple, safe, and quick procedure, which does not considerably increase the graft anoxia time. These conditions were present in the current investigation, and there was no morbity or mortality increase, which is in accordance with other observations presented in the literature [11,13].
In the postoperative of the transplantation, the tricuspid insufficiency secondary to the right ventricle dysfunction is accompanied by long-term survival reduction of the patients [10,19]. It was not possible to observe significant differences in the long-term mortality in this study, possibly due to small casuistic and short time of follow-up.
The association of tricuspid annuloplasty in the donor heart is an alternative that can reduce valvular insufficiency after transplantation, and it has the potential for long-term benefits. The outcomes of this research, even initial outcomes, are not distinguished from other experiences in the literature [11,13]. However, an increase in casuistic and postoperative follow-ups becomes necessary to raise the status of the procedure.
This is an initial study performed in a single center to evaluate the topic in question; the surgical team was divided in order to ease the technique's standardization. The patients were chosen for a non-random study, which will henceforth become compulsory procedure to gain a better understanding of the outcomes. Respecting the limitations previously mentioned, it can be concluded that the prophylactic tricuspid annuloplasty in the donor heart is reproducible and easy to perform. Furthermore, in the period under consideration, it does not increase mortality, and it reduces valvular insufficiency in the postoperative of cardiac transplantation with bicaval anastomosis, doing so without hindering the hemodynamic performance of the graft.
REFERENCES
1. Sahar G, Stamler A, Erez E, Ben-Gal T, Sagle A, Aravot D, et al. Etiological factors influencing the development of atrioventricular valve incompetence after heart transplantation. Transplant Proc. 1997;29(6):2675-6. [
MedLine]
2. Mishra PK. Trivial tricuspid regurgitation: is the impact really trivial? Eur J Cardiothorac Surg. 2006;29(4):634-5. [
MedLine]
3. Alharethi R, Bader F, Kfoury AG, Hammond ME, Karwande SV, Gilbert EM, et al. Tricuspid valve replacement after cardiac transplantation. J Heart Lung Transplant. 2006;25(1):48-52.
4. Williams MJ, Lee MY, DiSalvo TG, Dec GW, Picard MH, Palacios IF, et al. Biopsy-induced flail tricuspid leaflet and tricuspid regurgitation following orthotopic cardiac transplantation. Am J Cardiol. 1996;77(15):1339-44. [
MedLine]
5. Lower RR, Stofer RC, Shumway NE. Homovital transplantation of the heart. J Thorac Cardiovasc Surg. 1961;41:196-204. [
MedLine]
6. Aziz TM, Saad RA, Burgess MI, Campbell CS, Yonan NA. Clinical significance of tricuspid valve dysfunction after orthotopic heart transplantation. J Heart Lung Transplant. 2002;21(10):1101-8. [
MedLine]
7. Kuwaki K, Morishita K, Tsukamoto M, Abe T. Tricuspid valve surgery for functional tricuspid valve regurgitation associated with left-sided valvular disease. Eur J Cardiothorac Surg. 2001;20(3):577-82. [
MedLine]
8. Deleuze PH, Benvenuti C, Mazzucotelli JP, Perdrix C, Le Besnerais P, Mourtada A, et al. Orthotopic cardiac transplantation with direct caval anastomosis: is it the optimal procedure? J Thorac Cardiovasc Surg. 1995;109(4):731-7. [
MedLine]
9. Koch A, Remppis A, Dengler TJ, Schnabel PA, Hagl S, Sack FU. Influence of different implantation techniques on AV valve competence after orthotopic heart transplantation. Eur J Cardiothorac Surg. 2005;28(5):717-23. [
MedLine]
10. Kpodonu J, Massad MG, Geha AS. Surgical considerations in the correction of valve dysfunction following heart transplantation. Clin Transplant. 2005;19(5):694-7. [
MedLine]
11. Jeevanandam V, Russell H, Mather P, Furukawa S, Anderson A, Grzywacz F, et al. A one-year comparison of prophylactic donor tricuspid annuloplasty in heart transplantation. Ann Thorac Surg. 2004;78(3):759-66.
12. Brown NE, Muehlebach GF, Jones P, Gorton ME, Stuart RS, Borkon AM. Tricuspid annuloplasty significantly reduces early tricuspid regurgitation after biatrial heart transplantation. J Heart Lung Transplant. 2004;23(10):1160-2. [
MedLine]
13. Jeevanandam V, Russell H, Mather P, Furukawa S, Anderson A, Raman J. Donor tricuspid annuloplasty during orthotopic heart transplantation: long-term results of a prospective controlled study. Ann Thorac Surg. 2006;82(6):2089-95.
14. Sarsam MA, Campbell CS, Yonan NA, Deiraniya AK, Rahman AN. An alternative surgical technique in orthotopic cardiac transplantation. J Card Surg. 1993;8(3):344-9. [
MedLine]
15. Fiorelli AI, Stolf NA, Honorato R, Bocchi E, Bacal F, Uip D, et al. Later evolution after cardiac transplantation in Chagas' disease. Transplant Proc. 2005;37(6):2793-8. [
MedLine]
16. Billingham ME, Cary NR, Hammond ME, Kemnitz J, Marboe C, McCallister HA, et al. A working formulation for standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. The International Society for Heart Transplantation. J Heart Transplant. 1990;9(6):587-93. [
MedLine]
17. Mügge A, Daniel WG, Herrmann G, Simon R, Lichtlen PR. Quantification of tricuspid regurgitation by Doppler color flow mapping after cardiac transplantation. Am J Cardiol. 1990;66(10):884-7. [
MedLine]
18. Fiorelli AI, Stolf NAG, Bocchi EA, Bellotti G, Jatene AD. Comportamento da função do ventrículo esquerdo a longo prazo no transplante cardíaco ortotópico. Rev Bras Cir Cardiovasc. 1993;8(2):97-107.
19. Anderson CA, Shernan SK, Leacche M, Rawn JD, Paul S, Mihaljevic T, et al. Severity of intraoperative tricuspid regurgitation predicts poor late survival following cardiac transplantation. Ann Thorac Surg. 2004;78(5):1635-42. [
MedLine]
20. Aziz T, Burgess M, Khafagy R, Wynn Hann A, Campbell C, Rahman A, et al. Bicaval and standard techniques in orthotopic heart transplantation: medium-term experience in cardiac performance and survival. J Thorac Cardiovasc Surg. 1999;118(1):115-22. [
MedLine]
21. Park KY, Park CH, Chun YB, Shin MS, Lee KC. Bicaval anastomosis reduces tricuspid regurgitation after heart transplantation. Asian Cardiovasc Thorac Ann. 2005;13(3):251-4. [
MedLine]
22. Filsoufi F, Salzberg SP, Anderson CA, Couper GS, Cohn LH, Adams DH. Optimal surgical management of severe tricuspid regurgitation in cardiac transplant patients. J Heart Lung Transplant. 2006;25(3):289-93. [
MedLine]
23. Mielniczuk L, Haddad H, Davies RA, Veinot JP. Tricuspid valve chordal tissue in endomyocardial biopsy specimens of patients with significant tricuspid regurgitation. J Heart Lung Transplant. 2005;24(10):1586-90. [
MedLine]
24. Leyh RG, Jahnke AW, Kraatz EG, Sievers HH. Cardiovascular dynamics and dimensions after bicaval and standard cardiac transplantation. Ann Thorac Surg. 1995;59(6):1495-500. [
MedLine]

 All scientific articles published at www.rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at www.rbccv.org.br are licensed under a Creative Commons license