Objective: To evaluate the efficiency of L-glutamic acid to prevent calcification of glutaraldehyde bovine pericardium implanted in rats' subcutaneous tissues.
Methods: Fifty four Wistar rats were divided in six groups according to the type of the bovine pericardium implanted. At first, all pericardia were initially cross-linked with 0.5% glutaraldehyd (GDA) fixative for 72 h. In Group I, after the initial fixation, the pericardia were preserved in 0.2% GDA fixative until the implantation, whereas in Group II they were stocked in Paraben solution. In Groups III and IV, after the initial fixation in 0.5% GDA fixative, the pericardia were treated with 8% L-glutamic acid at pH 7.4 and 3.5, respectivelly, being subsequently stocked in Paraben solution. Groups V and VI were similar to III and IV, except for the concentration of L-Glutamic acid which was 0.8%. Explantation was done at 15, 30, and 60 days, and the specimens submitted to histological analysis with Hematoxylin and eosin (HE) and Von Kossa stains, besides calcium quantification with atomic spectrofotometry.
Results: Microscopic analysis demonstrated severe and progressive calcification in groups I, II, and III, whereas in groups IV, V, and VI calcification, when present, was mild and focal. Spectrofotomety confirmed these findings, revealing calcium contents of 1.93µg/mg of tissue at 60 days in the control group. Groups IV and VI showed the least calcium contents (0.063 e 0.066, respectively).
Conclusions: The use of L-glutamic acid in segments of bovine pericardium with glutaraldehyde fixative was effective in preventing the calcification when implanted in rats' subcutaneous up to 60 days.
Objetivo: Avaliar a eficácia do ácido L-glutâmico na prevenção da calcificação do pericárdio bovino implantado no subcutâneo de ratos.
Método: Utilizaram-se 54 ratos Wistar, distribuídos em seis grupos, de acordo com o segmento de pericárdio bovino implantado. Inicialmente, todos os pericárdios foram fixados com glutaraldeído 0,5% por 72h. No grupo I, após a fixação, o pericárdio foi preservado em glutaraldeído 0,2% até o implante. O grupo II foi estocado em solução de Paraben. No grupo III e IV, após a fixação inicial, os pericárdios foram tratados com ácido L-glutâmico 8% com pH 7,4 e 3,5, respectivamente, sendo em seguida estocados em Paraben. Os grupos V e VI foram semelhantes aos grupos III e IV, exceto pela concentração do ácido L-glutâmico que foi de 0,8%. Os explantes foram feitos com 15, 30 e 60 dias, e as amostras submetidas à análise histológica com hematoxilina-eosina e Von Kossa, além da mensuração de cálcio por espectofotometria de absorção atômica.
Resultados: A mensuração por espectofotometria de absorção atômica demonstrou aumento progressivo da calcificação nos grupos I, II e III, aos 15, 30 e 60 dias. Nos grupos IV, V e VI, os níveis de cálcio permaneceram sem alteração nos períodos estudados. A análise microscópica demonstrou calcificação progressiva nos grupos I, II e III. Nos grupos IV, V e VI, a calcificação, quando observada, foi focal e de grau leve.
Conclusão: O uso do ácido L-glutâmico em segmentos de pericárdio bovino, fixados pelo glutaraldeído, foi efetivo na prevenção da calcificação, quando implantados no subcutâneo de ratos por até 60 dias.
INTRODUCTION
Glutaraldehyde (GDA) fixation of valvar prostheses has been widely employed in the past three decades [1]. It is also used to promote the collagen stabilization and to reduce rejection [2]. Nevertheless, the calcification of valve cuspids teated with glutaraldehyde still represents the leading cause of implants failure [3, 4].
Some authors have suggested that the fixation of biological tissue by glutaraldehyde (GDA) fixative, by itself, can be a facilitating agent of the calcification because the gradual release of monomeric glutaraldehyde after the implant, from the disruption of polymeric glutaraldehyde links, turns out into cytotoxic effect, thus inhibiting the endothelial proliferation process by exposing cellular debris rich in phospholipids, which have been proven to work as primary sites of the calcification process due to the great molecule polarity of the monomeric glutaraldehyde [3-9].
In the light of this, several alternative chemical treatments have been proposed in order to reduce or delay the calcification process.
Experimental studies have demonstrated that the treatment of the bovine pericardium with L-glutamic acid previously fixed with glutaraldehyde improved its biocompatibility by neutralizing the toxic effects of the glutaraldehyde. Also, it reduced significantly the calcification process. Moreover, the acid pH favors the polymeric glutaraldehyde depolymerization easing the exit of non-linked monomeric molecules tissue [4, 8].
The usage of an effective method to produce the bioprostheses is desirable because it could raise the strength, therefore reducing the demand of reoperations, particularly in developing countries, in which the usage of the biological prostheses is more frequent.
The aim of the present study was to analyze the efficacy of L-glutamic acid in concentrations of 0.8% and 8%, pH values ranging from 3.5 (acid) to 7.4 (physiologic), in the prevention of the segments calcification of the bovine pericardium with glutaraldehyde fixative and implanted in the subcutaneous tissue of young rats.
METHODS
This study was carried out at the following Labs: Laboratory of Experimental Surgery of Operative Technique Discipline of the Pontifical Catholic University of Paraná (PUC-PR), Curitiba, PR, Brazil; Laboratório da Cardioprótese Ltda; Laboratory of Clinical Pathology of Santa Casa de Misericórida, Curitiba, PR; and at the Institute of Technology for Development (LACTEC), Curitiba, PR, Brazil. The experiment was approved by the Institutional Ethics Animal Research Committee of PUC-PR under registration NR 62.
Fifty-four male Wistar rats (Rattus norvegicus albinus, Rodentia mammalia), with a mean age and weight of 33 ± 3 days and 90 ± 4 g, respectively, were used in the experiment.
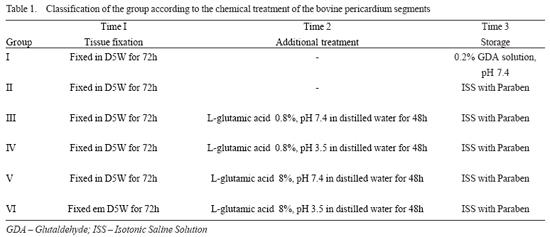
The animals were divided into 6 groups (I, II, III, IV, V, and VI), with nine animals each group, according to the chemical treatment of the bovine pericardium implanted (Table 1).
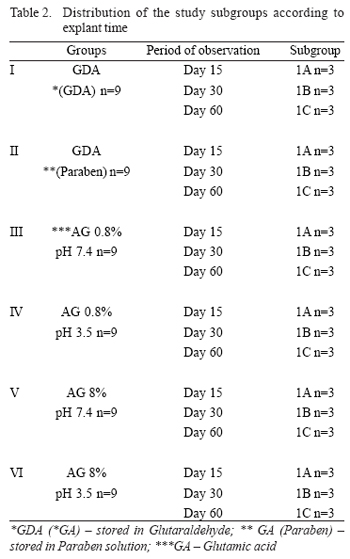
Each group was subdivided into 3 subgroups according to the time of postoperative outcome at the time of the animals' euthanasia to perform the explantation of the segments of the bovine pericardium within 15, 30, and 60 days (Table 2).
Acquisition of the bovine pericardium
The bovine pericardia were acquired from a slaughter-house at commercial slaughter age, just after the slaughter. The animals were dissected and free of pleural and mediastinal fat; they were washed and transported into an iced Ringer lactate solution at 4ºC until the laboratory where they were processed.
Chemical procedures
In all groups, the bovine pericardium segments were previously fixed in a 0.5% glutaraldehyde solution, in a buffer phosphate solution, with pH value of 7.4 for 72 hours, at room temperature (standard fixation). After this phase, the management of the bovine pericardium segments in different forms between the groups was carried out (Table 1).
Group I, or control group, received the conventional treatment used in commercial bioprostheses available, that is, after the initial fixation with 0.5% glutaraldehyde solution, the bovine pericardium segments were stored in a buffered solution with 0.2% glutaraldehyde solution. The pericardia from group II were fixed in the same way of that of the group I, differing from these only in their storage, which was performed in a Paraben solution. In group III, after the bovine pericardium segments were fixed in 0.5% glutaraldehyde solution, the pericardium segments were treated with a 0.2% glutaraldehyde buffered solution. The pericardia of group II were fixed in the same way of that of group I, differing this only in the storage, which was done in a Paraben solution. In group III, after being fixed with a 0.5% glutaraldehyde solution, the pericardium segments were treated with a 0.8% L-glutamic acid buffered solution, pH = 7.4, for 48 hours. Later they were stored in isotonic saline solution (SSI) with Paraben solution. In group IV, after the same fixation has been done, the pericardium segments were treated with a 0.8% L-glutamic acid solution, pH = 3.5, for 48 hours. Later, the segments were stored in SSI with Paraben solution. The groups V and VI were treated in a similar form. After the fixation, the pericardium segments were kept in a 8% L-glutamic acid solution with a different pH (group V, pH = 7.4 and group VI, pH = 3.5).
Operative technique
A 2-cm incision was performed in the region of back and a subcutaneous space was prepared to receive the bovine pericardium implant under the animal skin.
Postoperative observation
The rats were marked with a special skin pen marker and housed in appropriated cages. Each cage housed five animals. The rats were kept in a vivarium, being submitted to euthanasia within 15, 30, and 60 days with a lethal dose of thiopental sodium (400 mg/kg) administered intraperitoneally. Subsequently, a longitudinal incision was made in the region of back to remove implants to perform both a microscopic analysis and the measurement of calcium by flame atomic absorption spectroscopy.
Microscopic analysis
The explants were fixed in a 10% formalin solution and referred to microscopic analysis. The histological examination was performed by the same observer blind to the group to which the specimen belonged.
The assessment of dystrophic calcification was carried out by means of a 4 head microscope (American Optical with 8x objective) using hematoxylin and eosin stain, and classified in four levels according to the percentage of segment compromised by the calcification (Table 3).
Calcium measurement
The calcium quantitative determination was performed by atomic absorption spectroscopy with a 4100 Spectrometer (PerkinElmer Inc., Waltham, MA).
The quantity of total calcium concentration was expressed in milligrams per sample and extrapolated to µm/mg of preimplanted tissue. Thus, it was possible to estimate the amount of calcium per milligram of dry tissue as well as the host cellular infiltration.
Statistical analysis
The quantification of calcium by atomic absorption spectroscopy and the dystrophic calcification analysis variables were initially submitted to the assessment of their distribution through normality tests, coefficient of variation, and histogram analysis.
Both Kruskal-Wallis and Friedman tests and the analysis of variance (ANOVA) were used in order to evaluate the possible differences between the two groups as to the indices of dystrophic calcification, taking into consideration the asymmetric distribution of the variables and the absence of homoscedasticity between both groups.
All statistical tests were two-sided. It was taken into consideration that the differences could be distributed for both sides of the curve with a significance level of at least 5%. The sample size was estimated considering a type I error (alfa error) of 5% and a type II error (beta error) of 10%.
RESULTS
Analysis of calcification in the study groups by atomic absorption spectroscopy.
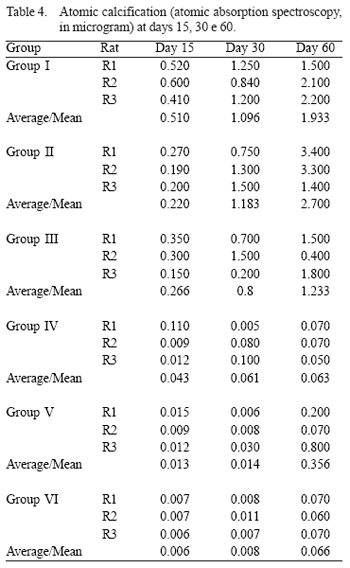
Group I or Control Group - It was observed an increasingly calcification based on the slaughtering day (Table 4).
Group II - It was also possible to observe the increasingly calcification over time. There was no statistically significant difference (p = 0.11) compared to group I (Table 4 and Figure 1).
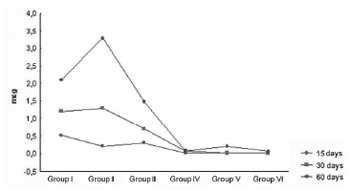
Fig.1 - Calcification within 60 days following the implant - microphotograph showing the bovine pericardium calcification levels according to the treatment regimen employed in groups I, II, III, IV, V, and VI, within 60 days of the implantation in the animals' subcutaneous tissues. (HE 40x)
Group III - The pericardia from group III also presented an increasingly calcification (Table 4).
Group IV - It was observed a significantly decreased pericardium calcification, even after 60 days in the animals' subcutaneous tissues. There was a statistically significant difference regarding group I (p = 0.001), group II (p = 0.01), and group III (p = 0.008) (Table 4).
Group V - It was observed significantly different calcification levels among the groups: group I (p = 0.04), group II (p = 0.001), and group III (p = 0.03); and a lower proportion when compared to group IV (p = 0.09) (Table 4).
Group VI - The results were as satisfying as the ones from group IV. There was no trend to calcification at day 60 (Table 4, Figure 1).
Microscopic analysis (histological) of the dystrophic calcification in the study groups
Two weeks (15 days) after the animals slaughtering
The microscopic analysis corroborated the data obtained by the atomic absorption spectroscopy. After 2 weeks, the pericardia of groups I, II, and III had already displayed dystrophic calcification, more markedly in the tissue central portion. Calcification was absent or had a minimum intensity, or was eminently focal in groups IV, V, and VI.
One month (30 days) after the animals slaughtering
One moth after the animals slaughtering it was observed, in a similar way to what was observed at 2 weeks after the animals slaughtering, that the calcification comprising higher percentage rates of tissue have occurred with a higher frequency in groups I, II, and III, whereas in groups IV, V, and VI the calcification occurred in its mild level (0-10% of the segment).
Two month (60 days) after the animals slaughtering
Two month (60 days) after the animals slaughtering, it was verified, in a similar way to what was observed at 2 and 4 weeks after the animals slaughtering, that the calcification comprising higher percentage rates of tissue occurred with a higher frequency in groups I, II, and III, while in groups IV, V, and VI it occurred in its mild level (0-10% of the segment) (Figure 2).

Fig. 2 - Microphotograph showing the bovine pericardium calcification levels according to the treatment regimen employed in groups I, II, III, IV, V, and VI, within 60 days of the implantation in the animals' subcutaneous tissues. (HE 40x)
The set of plots in Figure 3 illustrates the development in each group according to the time of animals' euthanasia, highlighting the progressive decrease of the dystrophic calcification extension of the pericardium segment regarding the treatment performed (Figure 3).

Fig. 3 - Plots showing the distribution of the dystrophic calcification level according to different treatment regimens applied to the pericardium segment and according to the time of animals' sacrifice
Leufauf et al.IONicardium segment regarding the treatment performed (Figure 3). [10] have analyzed the treatment effect with L-glutamic acid in bovine pericardia previously fixed with glutaraldehyde. They have observed that seeding "in vitro" of endothelial cells on untreated tissues resulted in the death of these cells in les than 48 hours, while in the tissues treated with glutamic acid there has been an adequate cellular proliferation, even though with a low complacence to the underlying tissue. In the same study, bovine pericardium tubular grafts were implanted in sheep carotid arteries. They have observed that in the bovine pericardia treated only with glutaraldehyde, the inner surface was coated by a fibrin layer with thrombus composed of platelets and macrophages, besides rare endothelial cells. In the tubular grafts treated with L-glutamic acid, there have been a partial reendothelization with extracellular matrix production, thus indicating a better biocompatibility of the treated tissue [10].
In a subsequent study, Grimm et al. [8] have analyzed the efficacy of the treatment with L-glutamic acid in preventing the calcification of bovine pericardia fixed with glutaraldehyde. Fragments of the pericardium implanted in the subcutaneous tissue of Spregue-Dawly rats for periods up to 63 days emphasized significant reduction not only from the inflammatory reaction, but also of the dystrophic calcification. In delayed explants (more than 60 days), the calcium level in the conventional pericardia was 1.69 µg/mg of tissue [8]. These values are similar to those found in the current study, while in those treated with L-glutamic acid was only 0.13 µg/mg of tissue [8]. These values are similar to the ones found in the current study in which the control group presented 1.93 µg of calcium per mg of tissue, while in bovine pericardia of groups IV and VI, where the treatment with L-glutamic acid was more effective, the calcium levels were 0.066 and 0.063 µg/mg of tissue in grafts explanted within 60 days. In groups III and IV, L-glutamic acid was used in the same concentrations, i.e., 0.8%, but with a different pH. It was observed that the outcomes were better in group IV (acid pH).
When the obtained results were analyzed, the L-glutamic acid in a buffered solution and in a pH of 7.4 (group V) were evaluated, it could be observed that within 60 days this group had a higher degree of calcification than that observed in groups IV and VI with a pH value of 3.5; However, group V presented better outcomes than that obtained in group III, in which the acid concentration of tenfold less (0.8%).
Grimm et al. [8], in their experiments also tested the L-glutamic acid in a buffered solution and in acid pH (3.5) and they concluded that the valves prepared with physiologic pH (8.4) favored a significant incorporation of the glutaraldehyde polymers in the tissue. These polymers are large, heavy molecules which can breakdown themselves and be used as a permanent reserve of toxic aldehydes. Thus, the L-glutamic acid in the physiologic pH impairs its anticalcifying potential because the acid-base chemical bind is unstable [8].
The benefit observed with the use of a more acid pH (3.5) probably results from two independent mechanisms: a) the acid medium during the exposure to the L-glutamic acid favors the built up of monomeric aldehydes which are smaller and lighter molecules, easing the tissue flush and removal; b) when occurs a chemical interaction between the acid and the aldehyde occurs a transformation into ester and acetate avoiding the release of the toxic aldehyde into the tissue.
In the current study it was evident the significant reduction of the calcification levels achieved with the treatment of the pericardium segment with L-glutamic acid.
Further studies must be carried out to make evident whether the L-glutamic acid anticalcifying action is maintained in bovine pericardium bioprostheses implanted in the circulatory (vascular) system.
CONCLUSIONS
The treatment of the bovine pericardium segments with 0.8% solution of L-glutamic acid with a pH of 3.5 fixed with glutaraldehyde was effective inpreventing the calcification when implanted in the young rats subcutaneous tissue up to 60 days. The treatment with 8% of L-glutamic acid with a pH of 3.5 also was effective; however, the L-glutamic acid in this concentration can be toxic to other tissues. Thus, to be produce commercially the cardiac prostheses have to be treated with a 0.8% solution of L-glutamic acid with a pH = 3.5.
REFERENCES
1. Hendriks M, Everaerts F, Verhoeven M. Alternative fixation of bioprostheses. J Long Term Eff Med Implants. 2001;11(34):163-83.
2. Carpentier A, Lemaigre G, Robert L, Carpentier S, Dubost C. Biological factors affecting long-term results of valvular heterografts. J Thorac Cardiovasc Surg. 1969;58(4):467-83. [
MedLine]
3. Nimni ME, Bernick S, Cheung DT, Ertl DC, Nishimoto SK, Paule WJ, et al. Biochemical differences between dystrophic calcification of cross-linked collagen implants and mineralization during bone induction. Calcif Tissue Int. 1988;42(5):313-20. [
MedLine]
4. Grabenwoger M, Grimm M, Eybl E, Leukauf C, Muller MM, Plenck H Jr, et al. Decreased tissue reaction to bioprosthetic heart valve material after L-glutamic acid treatment. A morphological study. J Biomed Mater Res. 1992;26(9): 1231-40. [
MedLine]
5. Schoen FJ, Levy RJ. Tissue heart valves: current challenges and future research perspectives. J Biomed Mater Res. 1999;47(4):439-65. [
MedLine]
6. Golomb G, Schoen FJ, Smith MS, Linden J, Dixon M, Levy RJ. The role of glutaraldehyde-induced cross-links in calcification of bovine pericardium used in cardiac valve bioprostheses. Am J Pathol. 1987;127(1):122-30. [
MedLine]
7. Schoen FJ, Levy RJ. Calcification of tissue heart valve substitutes: progress toward understanding and preventison. Ann Thorac Surg. 2005;79(3):1072-80. [
MedLine]
8. Grimm M, Eybl E, Grabenwoger M, Griesmacher A, Losert U, Bock P, et al. Biocompatibility of aldehyde-fixed bovine pericardium. An in vitro and in vivo approach toward improvement of bioprosthetic heart valves. J Thorac Cardiovasc Surg. 1991;102(2):195-201. [
MedLine]
9. Carpentier A, Blondeau P, Laurens P, Mancel P, Laurent D, Dubost C. Replacement of mitral and tricuspid valves by heterografts. Ann Chir Thorac Cardiovasc. 1968;7(1):33-8. [
MedLine]
10. Leukauf C, Szeles C, Salaymeh L, Grimm M, Grabenwoger M, Losert U, et al. In vitro and in vivo endothelialization of glutaraldehyde treated bovine pericardium. J Heart Valve Dis. 1993;2(2):230-5. [
MedLine]




 All scientific articles published at www.rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at www.rbccv.org.br are licensed under a Creative Commons license