INTRODUCTION
Heart failure (HF) is, today, effectively treated by medicinal therapy with a significant impact on survival [1-3]. However, mortality remains high especially in patients with severe ventricular dysfunction [4,5]. Sudden death (SD) and the progression of HF itself are the most frequent causes of death in this population [5]. In this context, the utilization of implantable cardioverter defibrillators (ICD) and cardiac resynchronization therapy (CRT) constitute great advances. Both interventions are independently effective in the reduction of mortality in patients with HF and severe ventricular dysfunction [6,7]. The technological evolution of these devices is impressive and currently we have the possibility of implanting an apparatus with associated ICD and CRT functions. Thus, the question 'how should we approach this type of patient?' emerges; should we indicate ICD, CRT or both?
In this review, the available evidence for the indication of ICD and CRT in isolation and in association will be discussed independently of the economic impact attributed to the implantation of these devices.
IMPLANTABLE CARDIOVERTER DEFIBRILLATOR
The physiopathology of HF involves the interaction between a generator of electrical instability with the induction of ventricular tachycardia, which degenerates to ventricular fibrillation in 80 to 85% of the cases [8]. The greatest cause of HF is coronary artery disease in association or not with myocardial infarction. This is responsible for 75% of the events [8]. Since the first report on ICD implantation in 1980, treatment of ventricular tachyarrhythmias has undergone great transformations [9]. Initially utilized in survivors of HF, ICD started to be used as a preventive therapy for high risk patients of arrhythmic events.
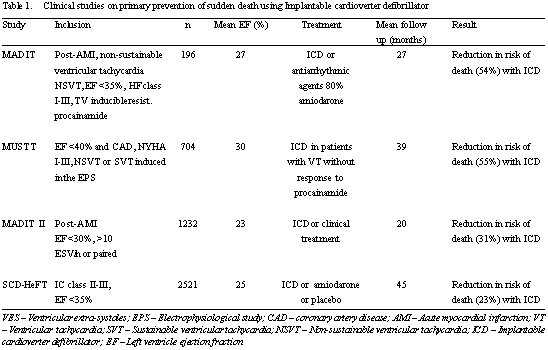
The Canadian Implantable Defibrillator Study (CIDS) [10], the Anti-arrhythmic versus Implantable Defibrillators Study (AVID) [11] and the Cardiac Arrest Study Hamburg (CASH) were simultaneously carried out evaluating similar populations with histories of reverted cardiorespiratory arrest or episodes of hemodynamically instable ventricular tachycardia. The studies showed a reduction in 30% of mortality and ICD became the therapy of choice in this group of patients [13]. The most detailed analysis of these studies enabled the identification of reduced ejection fractions (EF) (< 35%) and not the presence of arrhythmias as the important risk marker for HF [14].
The initial primary prevention trials were achieved with patients with reduced EF and inducible ventricular arrhythmias in electrophysiologic studies [15,16]. The Multicenter Automated Defibrillator Implantation Trial (MADIT) was the first large-scale study of primary prophylaxis and demonstrated a 54% reduction in mortality of ischemic patients who received ICD when compared to clinical treatment alone [15]. Similar to previous reports, reduced EFs were the important risk marker, whilst the electrophysiological study did not prove to be an effective independent prognostic marker for ventricular arrhythmias [15,16].
Based on the results of MADIT, MADIT II was designed utilizing reduced EF (< 30%) in isolation as the inclusion criterion for ICD or pharmacological treatment in patients with acute myocardial infarction of more than 30 days [17]. A reduction of 31% in the mortality risk was observed in the group treated with ICD, therewith increasing the indication of this device.
All the information until 2004 was based on patients with ischemic disease and ventricular dysfunction. The Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation study (DEFINITIVE) randomized 448 patients with dilated non-ischemic heart disease, EF < 36%, the frequent presence of ventricular extra-systoles or non-sustained ventricular tachycardia for treatment with optimized medical therapy (OMT) - diuretics, conversion enzyme inhibitors, b-blockers and spirolactone - and OMT plus ICD [18]. After a mean follow-up period of 29 months, there was a non-significant statistical trend of reducing the mortality rate for all causes (p-value = 0.08) and a reduction in the episodes of SD (p-value = 0.006) in the ICD group. Subsequently, the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) was published which presented greater statistical weight to evaluate differences in the mortality rates from ICD in non-ischemic patients due to the larger sample size [6].
This study randomized 2521 patients with HF in functional classes II or III and EFs < 35% for ONT + placebo (847 patients), OMT + amiodarone (845 patients) or OMT + ICD (829 patients). A total of 52% of the patients had ischemic myocardiopathy and 48% had non-ischemic myocardiopathy. The primary objective of the study was to evaluate the cause of mortality. In an average follow-up period of 45 months, therapy with ICD resulted in a reduction in 23% (NNT=14) of mortality by any cause with ODDS ratio (OR) of 0.77 (97.5% confidence interval: 0.62 - 0.96) in comparison to OMT. Amiodarone presented a null effect in the mortality in relation to the placebo with an OR of 1.06 (97.5% confidence interval: 0.86-1.30). An analysis of the subgroups demonstrated a similar effect in the reduction of mortality with ICD in ischemic and non-ischemic populations. The prophylactic studies of SD utilizing ICD are summarized in Table 1.
With the publication of MADIT II and SCD-HeFT, ICD took an important role in primary prophylaxis of SD in patients with HF and EFs < 35%, independent of etiology.
CARDIAC RESYNCHRONIZATION THERAPY
Treatment with ICD presented a great impact in the reduction of mortality due to SD in patients with HF, although ICD may give a greater benefit in distinct populations. In the MERIT-HF study, while 64% of the patients with class II HF suffered SD, the progression of HF was responsible for 68% of the deaths in patients with Class IV HF [5].
CRT was developed with the aim of reverting the adverse effects of cardiac desynchronization on the left ventricular function and on the functional capacity of patients with advanced HF and widened QRS intervals (> 120 ms) [19]. The electromechanical delay, in this clinical setting, results in interventricular desynchronization between the right and left ventricles and in the left ventricular contractions (intraventricular desynchronization). The latter most frequently results in a block of the left branch, altering the contractile synergism pattern. Hence, there is a loss in systolic function, reducing the heart output, increasing the final diastolic volume, aggravating mitral regurgitation and causing an abnormal movement of the septal wall [20].
A large part of the information available on the clinical benefits of CRT is from recent clinical studies. The MIRACLE [21], MUSTIC [22] and CONTAK CD [23] trials demonstrated that CRT determines an improvement in the functional class of HF patients, tolerance to exercise (6-minute walking test and VO2 peak), reduction in the hospitalization rate due to HF and improvement in the quality of life evaluated using the Minnesota questionnaire. Nevertheless, none of these studies demonstrated a reduction in the mortality rate with CRT, with no statistical significance seen evaluating this outcome.
There are some limiting factors to be discussed in relation to CRT. The first is in respect to the procedure technique which presented a failure rate of 8 to 13% in the endocardial implantation of the left ventricular electrode due to anatomic variations [24]. Additionally, we must remember that, even after a technically effective procedure, around 30% of the patients may not present with a clinical improvement [21-23], which may be explained by the limitations of the current selection criteria. Although the electrocardiographic finding of the widened QRS complex is suggestive of desynchronization, probably it is not an independent response predictor to CRT [25].
The Cardiac Resynchronization - Heart Failure (CARE-HF) study was the first designed to test the hypothesis that CRT in isolation improves survival in HF [7]. A total of 813 patients in classes III and IV HF, EF £ 35%, QRS interval > 120 ms, associated to criteria of ventricular desynchronization (QRS intervals between 120 and 149 ms) were investigated. The patients were randomized for medical therapy or CRT alone, with primary results of death by any cause. The criteria of desynchronization utilized were aortic pre-ejection delay (> 140 ms), delay in the interventricular mechanical contraction (> 40 ms), or delay in the activation of the postero-lateral wall of the left ventricle. During a mean follow-up period of 29 months, the study demonstrated a reduction in the total mortality rate in the CRT group with an OR of 0.63 (95% confidence interval: 0.51 - 0.77). To be included in the study all the patients were in sinus rhythm and more than 90% of the sample presented with class III HF.
A significant difference from the CARE-HF study in respect to the previous studies was related to the selection criteria. The demands of the cardiac desynchronization criteria confirmed by echocardiography in patients with a QRS complex interval between 120 and 149 ms, possibly reduced the number of patients that did not respond to CRT.
ICD, CRT OR BOTH?
After the publication of the SCD-HeFT and CARE-HF studies, there was an significant increase in the number of candidates for electric therapy utilizing these devices [6,7]. These studies included patients with similar characteristics except, obviously, the necessity of ventricular desynchronization for CRT. However, 50% and 41% of the patients included in the MADIT II and SCD-HeFT studies, respectively had QRS intervals > 120 ms and both demonstrated a greater benefit of ICD in these subgroups. Thus, the response to the question of when ICD in isolation should be indicated for patients with HF is simple: in the presence of classes II-III HF, EF < 35% and the absence of a widened QRS complex. This indication can be extended to patients in functional class I, EF < 30% and prior myocardial infarction [17]. Even so, there are clinical settings where there may be doubts, such as:
"Should we always associate ICD to patients with indication for CRT?"
"Should we always associate CRT to patients with indication of ICD when there is ventricular desynchronization?"
Until now, there have been no studies specifically to answer these questions. The COMPANION study (Comparison of medical therapy, pacing and defibrillation in heart failure trial) randomized 1520 patients in classes III and IV HF, EF < 35% and QRS interval > 120 ms for isolated OMT or in association with CRT, or associated to CRT + ICD [24]. The primary objective was to evaluate the difference in relation to combined outcomes of death by any cause and the number of hospitalizations, and the secondary result was death by any cause. Compared to OMT, CRT in isolation and CRT with ICD gave a reduction in the primary outcome with OR 0.81 (95% confidence interval: 0.69 - 0.96) and 0.80 (95% confidence interval: 0.68 - 0.95). There was a reduction of 36% in mortality in the CRT + ICD group in relation to the OMT group (p-value = 0.003), whilst the CRT group showed a non-significant tendency for the same outcome with a reduction of 24% (p-value = 0.059) in relation to the OMT group. There was no significant difference in respect to primary and secondary outcomes when the CRT and CRT + ICD groups were compared with the primary outcome equal in both groups (56%).
However, it is important to stress that the COMPANION study was not designed to identify differences between the CRT and CRT + ICD groups, but to compare both these groups with the OMT group. Another important aspect is related to the difference in the dissynchrony criteria utilized in the COMPANION study in respect to CARE-HF [7,24]. Thus, although the COMPANION study is the only work that compares CRT in isolation with CRT + ICD, it is incapable of giving a definitive answer as to whether there are advantages in this association as it does not have statistical power to detect this difference.
The evaluation of the HF functional class may be a significant aspect. Whilst the SCD-HeFT study randomized patients in classes II and III HF, the CARE-HF study included patients in classes III and IV HF. In SCD-HeFT, the majority of the patients (70%) presented in functional class II. In the analysis of the subgroups, the functional class II patients presented with better results with the implantation of the ICD. Hence, for patients with EF < 35% and HF class II, there is no evidence of reduction in mortality with CRT yet and we believe there is no conflict in the indication of ICD in isolation, independent of the QRS interval observed on the electrocardiogram. On the other hand, in patients in HF class IV, EF < 35% and criteria of dissynchrony, it seems prudent to indicate CRT in isolation due to the lack of evidence showing the benefit of ICD in this population.
We also know that the progression of ventricular dysfunction is the most frequent cause of death in this population, making this decision less controversial [5]. Nevertheless, the great variability of HF functional class may make the choice of these criteria in the indication of specific devices, inconsistent. Additionally, more than 90% of the sample of patients enrolled in the CARE-HF study presented in functional class III [7]. The basic characteristics of the populations of the CARE-HF, SCD-HeFT and COMPANION studies are summarized in Table 2.

Another factor that deserves mention is the consequences of artificial heart pacing. It is well known that CRT sustainably increases the absolute value of EF by 2 to 10% in patients with ventricular dissynchrony [20-22]. It is also known that only the patients with EF d" 35% present with a proven benefit with the prophylactic implantation of an ICD - what would happen with a patient in HF class II with an EF < 30% - a patient with criteria of inclusion in the SCD-HeFT study for example - and ventricular dissynchrony, who was submitted to CRT with an improvement in the EF to 40%? This patient is not part of the group indicated for primary prophylaxis with ICD and we still do not know what the benefit of ICD in this specific population would be. On the other hand, it seems evident that on implanting an ICD in a patient with HF and diminished EF, we should avoid the use of the ICD pacemaker function by means of adequate programming and be careful when using negative chronotropic drugs. Artificial pacing of an electrode from the right ventricle apex may cause the same electromechanical lack of synergism that is caused by a block of the left branch [26]. The excessive use of the pacemaker function in the ICD group was one of the justifications presented by the authors of the MADIT II study for the lack of improvements in the survival rate in the first 9 months of follow up and the greater number of hospitalizations in the ICD group in relation to the OMT group [17].
CONCLUSION
The treatment of heart failure underwent significant improvements over the last five years in relation to the use of ICD and CRT in isolation. However, the information available in respect to the association of these two forms of therapy is still scarce. Hence, we should consider each individual case of ICD associated to CRT, reporting the evidence available, while waiting to see the results of the next studies on this theme.
REFERENCES
1. Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial Lancet. 2001;357(9266):1385-90.
2. Pfeffer MA, Braunwald E, Moyé L, Basta L, Brown EJ Jr, Cuddy TE et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327(10):669-77.
3. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure: Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709-17.
4. Packer M, Fowler MB, Roecker EB, Coats AJ, Katus HA, Krum H et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) study. Circulation. 2002;106(17):2194-9.
5. MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF). Lancet. 1999;353(9169):2001-7.
6. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). N Engl J Med. 2005;352(3):225-37.
7. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. Cardiac Resynchronization - Heart Failure (CARE-HF) Study Investigators. N Engl J Med. 2005;352(15):1539-49.
8. Myerburg RJ, Castellanos A. Cardiac arrest and sudden cardiac death. In: Braunwald E, Zipes DP, Libby P, eds. Heart disease: a textbook of cardiovascular medicine. 6th ed. Philadelphia:WB Saunders;2001. p.890-931.
9. Mirowiski M, Reid PR, Mower MM, Watkins L, Gott VL, Schauble JF et al. Termination of malignant ventricular arrhythmias with an implanted automatic defibrillator in human beings. N Engl J Med. 1980;303(6):322-4.
10. Connolly SJ, Gent M, Roberts RS, Dorian P, Roy D, Sheldon RS et al. Canadian Implantable Defibrillator Study (CIDS): a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation. 2000;101(11):1297-302.
11. The Antiarrhythmics Versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337(22):1576-83.
12. Kuck KH, Cappato R, Siebels J, Ruppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: the Cardiac Arrest Study Hamburg (CASH). Circulation. 2000;102:748-54.
13. Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmias. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335(26):1933-40.
14. Connolly SJ, Hallstrom AP, Cappato R, Schron EB, Kuck KH, Zipes DP et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs Implantable Defibrillator study. Cardiac Arrest Study Hamburg. Canadian Implantable Defibrillator Study. Eur Heart J. 2000;21(24):2071-8.
15. Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335(26):1933-40.
16. Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341(25):1882-90.
17. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. Multicenter Automatic Defibrillator Implantation Trial (MADIT II). N Engl J Med. 2002;346(12):877-83.
18. Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350(21):2151-8.
19. Gregoratos G, Abrams J, Epstein AE, Freedman RA, Hayes DL, Hlatky MA et al. ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices -summary article: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE Committee to Update the 1998 Pacemaker Guidelines). J Am Coll Cardiol. 2002;40(9):1703-19.
20. Sogaard P, Egeblad H, Kim WY, Jensen HK, Pedersen AK, Kristensen BO et al. Tissue Doppler imaging predicts improved systolic performance and reversed left ventricular remodeling during long-term cardiac resynchronization therapy. J Am Coll Cardiol. 2002;40(4):723-30.
21. Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346(24):1845-53.
22. Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344(12):873-80.
23. Auricchio A, Stellbrink C, Sack S, Block M, Vogt J, Bakker P et al. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol. 2002;39(12):2026-33.
24. Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. N Engl J Med. 2004;350(21):2140-50.
25. Doshi RN. Optimizing resynchronization therapy: can we increase the number of true responders? J Cardiovasc Electrophysiol. 2005;16(suppl 1):S48-51.
26. Ellenbogen KA, Thames MD, Mohanty PK. New insights into pacemaker syndrome gained from hemodynamic, humoral and vascular responses during ventriculo-atrial pacing. Am J Cardiol. 1990;65(1):53-9.


 All scientific articles published at www.rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at www.rbccv.org.br are licensed under a Creative Commons license